Graham’s Law of Diffusion
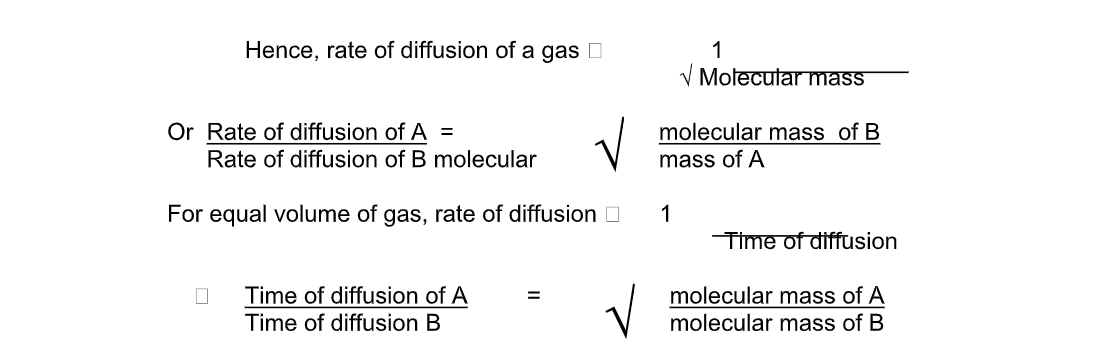
Graham’s law of diffusion relates the rate of diffusion of a gas to its density
It states that the rate of diffusion of a gas at constant temperature and pressure is inversely proportional to the square root of its density
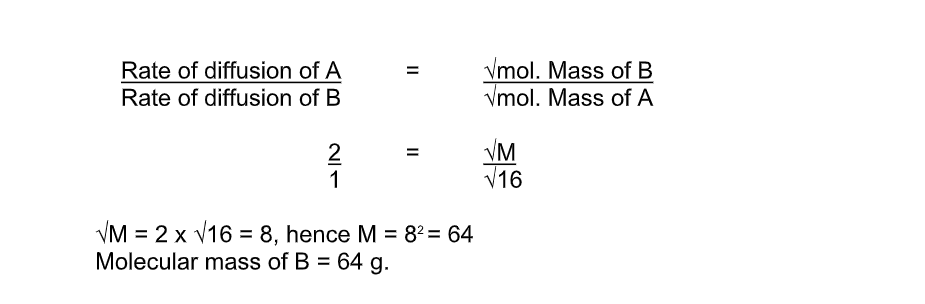
Evaporation, boiling and kinetic theory
On heating particles gain kinetic energy and move faster
In evaporation and boiling the highest kinetic energy molecules can ‘escape’ from the attractive forces of the other liquid particles
The particles lose any order and become completely free to form a gas or vapour
Energy is needed to overcome the attractive forces in the liquid and is taken in from the surroundings
This means heat is taken in, so evaporation or boiling are endothermic (require energy input) processes
If the temperature is high enough boiling takes place
Boiling is rapid evaporation anywhere in the bulk liquid and at a fixed temperature called the boiling point and requires continuous addition of heat
The rate of boiling is limited by the rate of heat transfer into the liquid
Evaporation takes place more slowly at any temperature between the melting point and boiling point, and only from the surface, and results in the liquid becoming cooler due to loss of higher kinetic energy particles
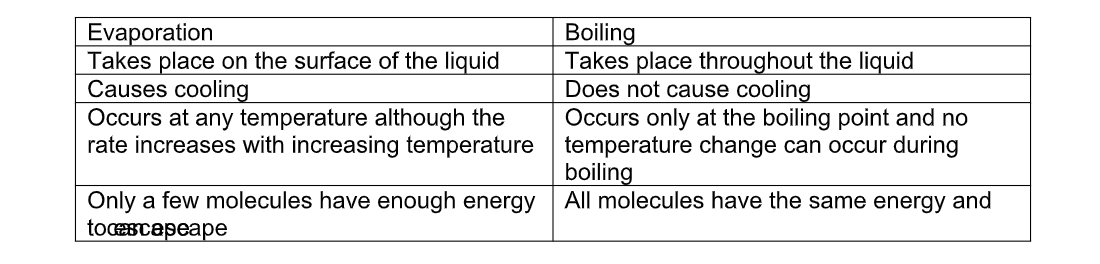
Differences between evaporation and boiling
Factors that affect the rate of evaporation
Evaporation occurs at all temperatures at the surface of the liquid
It happens more rapidly when:
i) The temperature is higher, since then more molecules in the liquid are moving fast enough to escape from the surface,
ii) The surface area of the liquid is large so giving more molecules a changes to escape because more are near the surface, and
iii) Wind or draught is blowing over the surface carrying vapour molecules away from the surface thus stopping them from returning to the liquid and making it easier for more liquid molecules to break free
Kinetic Theory and Gas Laws
Due to the kinetic theory we begin to understand why gases exert pressure
The molecules of a gas are far apart and in continuous random motion, colliding with each other and with the walls of the vessel in which the gas is held
The molecules have mass, so they have energy hence they exert force on each collision and hence pressure
If the temperature of the gas is increased at constant volume, the molecules gain more energy and move faster, hitting the walls with more force and exerting greater pressure
If the volume of the gas is increased at constant temperature, the molecules have more space in which to move
The frequency of collisions decreases reducing the pressure
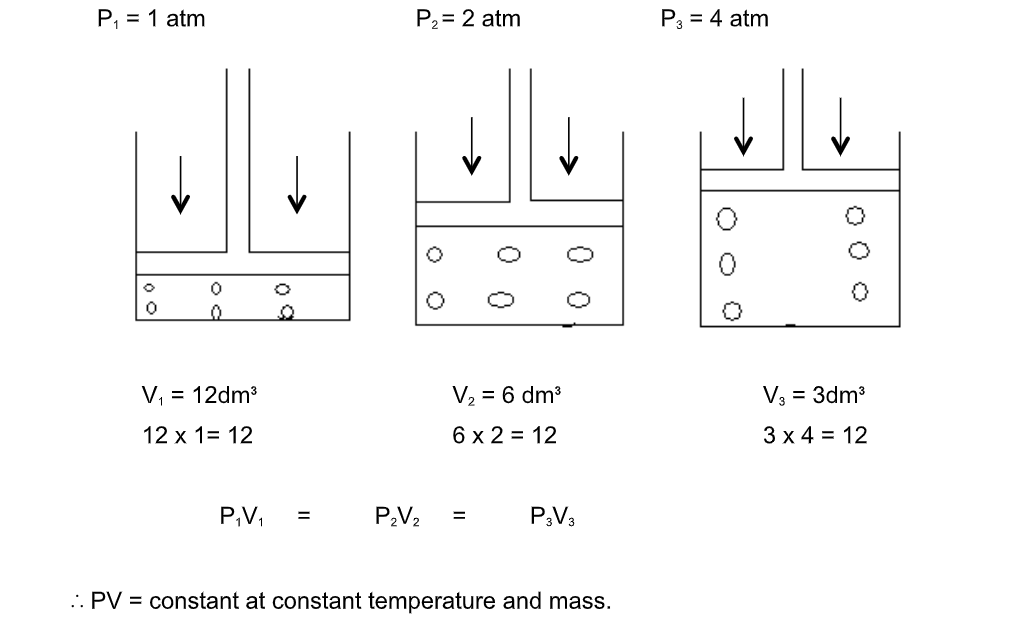
Boyle’s law
The pressure of a fixed mass of gas is inversely proportional to its volume if its temperature is kept constant
Consider a gas trapped in a container as shown
The mass, hence number of moles are constant and do not change during the course of the investigation
The piston is frictionless and moves smoothly without allowing the gas to escape
When the pressure changes, the volume of the gas changes as shown
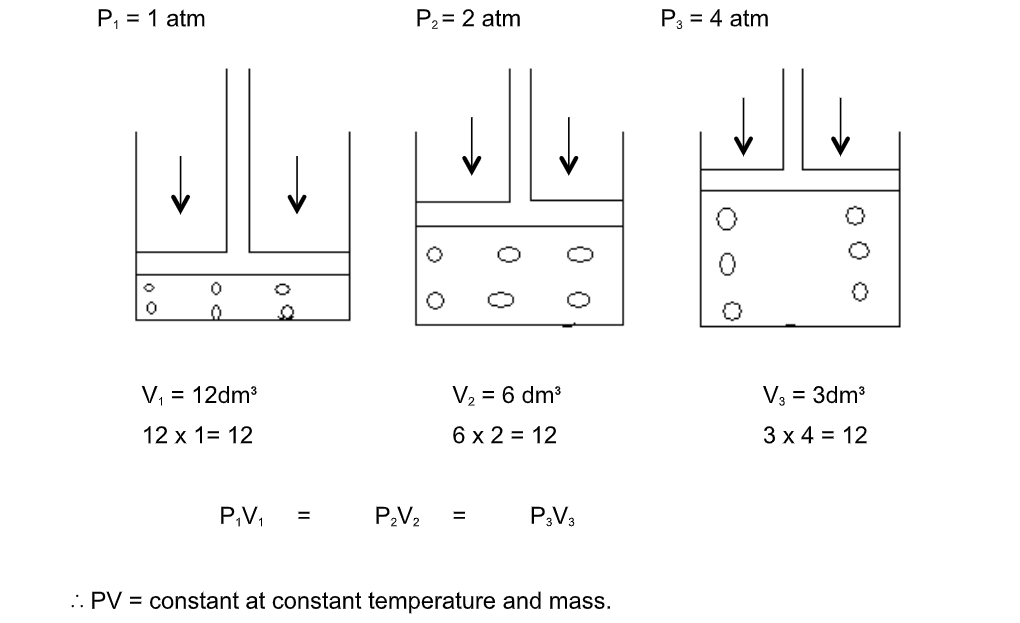
Graphical representation of Boyle’s law
Graph between P & V at constant temperature is a smooth curve known as “parabola”
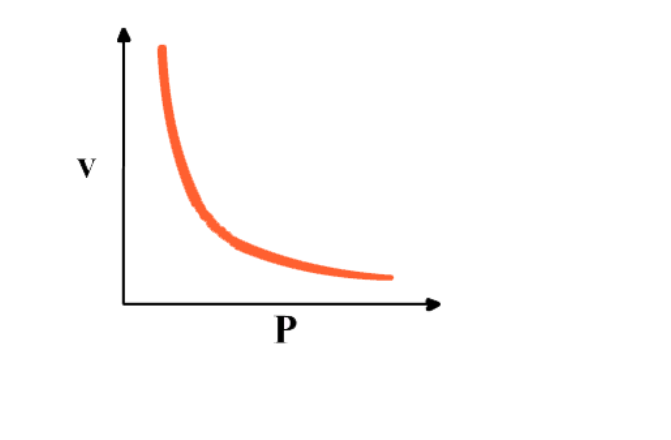
Graph between 1/P & V at constant temperature is a straight line
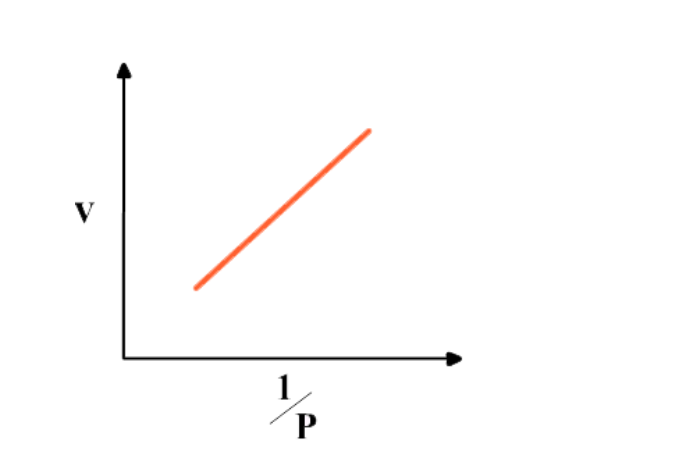
If pressure, p is doubled, the volume is halved
That is, p is inversely proportional to V
In symbols
P ∝ 1/V or p = constant X 1/V
PV = constant
p1V1 = p2V2 = constant.This is Boyle’s law
Charles’ Law
The volume of a fixed mass of gas is directly proportional to its absolute temperature if the pressure is kept constant
We can then say that the volume V is directly proportional to the absolute temperature T, ie doubling T doubles V, etc Therefore
V ∝ T or V = constant X T
Or V/T = Constant
Volume V1 = 1dm3 V2 = 2dm3 V3= 3 dm3
Temperature (OC) 0 ºC 273 ºC 546 ºC
Temp (K) 273 K 546K 819K
Volume/Temp 1/273 2/546 3/819
Equation ∴ V1/T1 = V2/T2 = V3/T3
∴ V/T = constant
Graphical representation
Graph between Volume and absolute temperature of a gas at constant pressure is a “straight line”
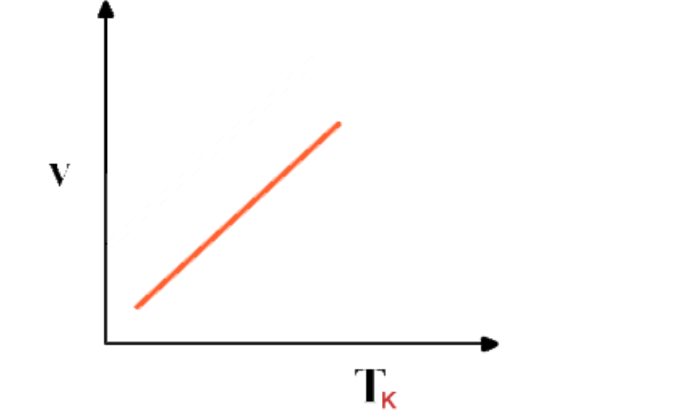
Absolute scale of temperature or absolute zero
If the graph between V and T is extrapolated, it intersects T-axis at -273
16 0C
At -273
16 0C volume of any gas theoretically becomes zero as indicated by the graph
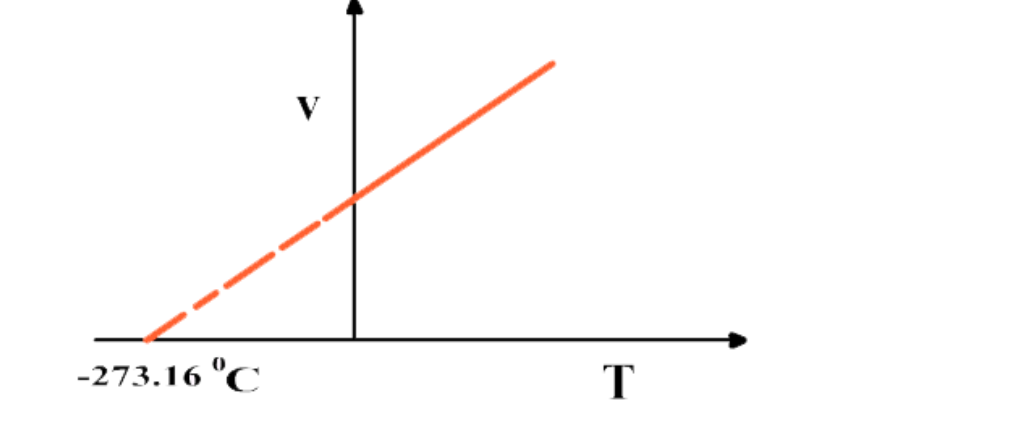
But practically volume of a gas can never become zero
Actually no gas can achieve the lowest possible temperature and before -273.16 0C all gases are condensed to liquid
This temperature is referred to as absolute scale or absolute zero
At -273.16 0C all molecular motions are ceased
This temperature is called Absolute Zero
Degrees on this scale are called Kelvin’s and are denoted by K while θ stands for a Celsius scale temperature
They are exactly the same size as Celsius degrees
Since –273 0C = 0K, conversions from 0C to K are made by adding 273 T = 273 + θ
0 0C = 273K
15 0C = 273 + 15 = 288K
The letter T represents Kelvin or absolute temperatures and θ stands for a Celsius scale temperature
Pressure law
The pressure of a fixed mass of gas is directly proportional to its absolute temperature if the volume is kept constant
p ∝ T or p = constant X T
Or p/T = Constant
The three equations can be combined giving
Pv = constant
T For cases in which p, V and T all change from say p1, V1 and T1 to p2, V2 and T2, then P1V1 = p2V2 T1 T2
Gases and the Kinetic Theory
The kinetic theory can explain the behaviour of gases
(a) Causes of gas pressure
All the molecules in a gas are in rapid motion, with a wide range of speeds, and repeatedly hit the walls of the container in huge numbers per second
The average force and hence the pressure they exert on the walls is constant since pressure is force on unit area
(b) Boyle’s law
If the volume of a fixed mass of gas is halved by halving the volume of the container, the number of molecules per cm3 will be doubled
There will be twice as many collisions per second with the walls, i.e the pressure is doubled
This is Boyle’s law
(c) Temperature
When a gas is heated and its temperature rises, the average speed of its molecules increases
If the volume of the gas is to remain constant, its pressure increases due to more frequent and more violent collisions of the molecules with the walls
If the pressure of the gas is to remain constant, the volume must increase so that the frequency of collisions does not
13.0.0 The Mole: Formulae and Chemical Equations
Mole Concept
What is a Mole?
A mole is a word which represents a number
Like the word “dozen” represents the number 12, so “mole” represents the number 6 x 1023
This number is also called Avogadro’s number
It is a very very big number (6 followed by 23 zeros)
In the same way that you can have a dozen atoms, or cars, or apples, so you can also have a mole of atoms, or cars, or apples
Needless to say, chemists are concerned with atoms, ions, molecules and compounds
A mole is defined as the number of atoms in exactly 12 grams of 12C (carbon twelve)
The number is called Avogadro’s number
In the above definition, 12 is the mass number of carbon
So, one mole of carbon atoms has a mass of 12 grams
The relative atomic mass, which can be written as Ar or RAM, is the number just above the element in the periodic table Relative atomic mass(r.a.m) and moles
Just as one mole of carbon atoms has a mass of 12 g,
so the mass of one mole of the atoms of any element
is its “relative atomic mass” in grams
For example, look up the relative atomic mass of sodium (Na), (the larger number above it in the periodic table)
One mole of sodium has a mass of 23 g
One mole of helium has a mass of 4 g,
One mole of neon has a mass of 20 g,
One mole of magnesium has a mass of 24 g, One mole of calcium has a mass of 40 g
This is easy for elements which exist as atoms
As discussed ealier, the number of protons added to the number of neutrons is known as the relative atomic mass
This is the mass of 1 mol of an atom relative to the mass of 1 mol of C atoms that have 6 protons and 6 neutrons, which is taken to be 12.00 g
However there are complications due to isotopes and so very accurate atomic masses are not whole numbers
Isotopes are atoms of the same element with different masses due to different numbers of neutrons
The very accurate atomic mass scale is based on a specific isotope of carbon, carbon-12, 12C = 12.0000 units exactly, for most purposes C = 12 is used for simplicity
The strict definition of relative atomic mass (Ar) is that it equals average mass of all the isotopic atoms present in the element compared to 1/12th the mass of a carbon-12 atom
Examples of relative atomic mass calculations
Example 1: chlorine consists of 75% chlorine-35 and 25% chlorine-37
Think of the data based on 100 atoms, so 75 have a mass of 35 and 25 atoms have a mass of 37
The average mass = [ (75 x 35) + (25 x 37) ] / 100 = 35.5
So the relative atomic mass of chlorine is 35.5 or Ar(Cl) = 35.5
What about elements which exist as molecules or compounds?
Relative formula mass of a compound (R.F.M):
To calculate the mass of one mole of a compound, the number of each type of atom in the compound is multiplied by that atoms relative atomic mass and all those numbers added together
This value is called the relative formula mass (or relative molecular mass or molar mass) of a compound
Notice that r.f.m, r.m.m, or Mr have no units because they are ratios
The molar mass is obtained from r.f.m, r.m.m, or Mr by simply adding g (grams)
If all the individual atomic masses of all the atoms in a formula are added together you have calculated the relative formula mass (for ionic compounds) or molecular mass (for covalent elements or compounds), Mr. can be used for any element or compound
Whereas relative atomic mass (above) only applies to a single atom, anything with at least two atoms requires the term relative formula/molecular mass
The most common error is to use atomic/proton numbers instead of atomic masses, unfortunately, except for hydrogen, they are different!
Examples of formula/molecular mass calculations:
Example 1:
The diatomic molecules of the elements hydrogen H2 and chlorine Cl2
relative atomic masses, Ar: H = 1, Cl = 35.5
Formula masses, Mr, are H2 = 2 x 1 = 2, Cl2 = 2 x 35.5 = 71 respectively
Example 2:
The compound calcium hydroxide Ca (OH)2(ionic)
Relative atomic masses are Ca=40, H=1 and O=16
Mr = 40 + 2 x (16+1) = 74
(2) Mass from amount:
The key mathematical equation needed here is –
mass (g) = relative formula mass (g mol-1) x amount (mol)
Using the triangular relationship from above if the mass section is covered over then the amount multiplied by the relative formula mass gives the mass
Example
(i) What is the mass of 0.25 mol of NaCl?
0.25 mol of NaCl = 58.5 g mol-1 × 0.25 mol
= 14.63 g
(ii) What is the mass of 3 mol of Al2(SO4)3?
3 mol of Al2(SO4)3 = 342 g mol-1 × 3 mol
= 1026 g or 1.026 kg
(3) Amount from mass :
The key mathematical equation needed here is –
amount (mol) = mass (g) / relative formula mass (g mol-1)
Using the triangular relationship from above if the amount section is covered over then the mass divided by the relative formula mass gives the amount –
Exemplar calculations –
(i) What amount is 117 g of NaCl?
117 g of NaCl = 117 g / 58
5 g mol-1 = 2 mol
(ii) What amount is 68
4 g of Al2(SO4)2?
68
4 g of Al2(SO4)3 = 68.4 g / 342 g mol-1 = 0.2 mol
(4) Molar mass from mass and amount:
The key mathematical equation needed here is –
Relative formula mass (g mol-1) = mass (g) / amount (mol)
Using the triangular relationship from above if the molar mass section is covered over then the mass divided by the amount gives the relative formula mass
Exemplar calculation
What is the molar mass of a compound for which 0.2 mol of it has a mass of 42 g?
molar mass of compound = 42 g / 0.2 mol
= 210 g mol-1
Every mole of any substance contains the same number of the defined species
The actual particle number is known and is called the Avogadro Constant and is equal to 6
023 x 1023 ‘defined species’ per mole
This means there are that many atoms in 12g of carbon (C = 12) or that many molecules of water in 18g (H2O = 1+1+16 = 18, H = 1; O = 16)
Note
Relative is just a number based on the carbon-12 relative atomic mass scale
Molar mass is a term used to describe the mass of one mole i.e the relative atomic/formula/molecular mass in grams (g)
Examples:
Example 1:
1 mole of ammonia, NH3, Consists of 1 mole of nitrogen atoms combined with 3 moles of hydrogen atoms
Or you could say 2 moles of ammonia is formed from 1 mole of nitrogen molecules (N2) and 3 moles of hydrogen molecules (H2)
Example 2:
1 mole of aluminium oxide, Al2O3, consists of 2 moles of aluminium atoms combined with 3 moles of oxygen atoms (or 1.5 moles of O2 molecules)
For calculation purposes learn the following formula for ‘Z’ and use a triangle if necessary
(1) mole of Z = g of Z / atomic or formula mass of Z,
(2) or g of Z = mole of Z x atomic or formula mass of Z
(3) or atomic or formula mass of Z = g of Z / mole of Z
where Z represents atoms, molecules or formula of the particular element or compound defined in the question
Example 1: How many moles of potassium ions and bromide ions in 0.25 moles of potassium bromide?
1 mole of KBr contains 1 mole of potassium ions (K+) and 1 mole of bromide ions (Br-)
So there will be 0.25 moles of each ion
Example 2: How many moles of calcium ions and chloride ions in 2.5 moles of calcium chloride?
1 mole of CaCl2 consists of 1 mole of calcium ions (Ca2+) and 2 moles of chloride ion (Cl-)
So there will be 2
5 x 1 = 2
5 moles of calcium ions and 2.5 x 2 = 5 moles chloride ions
mass of NaCl formed = moles x formula mass = 0
4 x 58.5 = 23.4g NaCl
Using the Avogadro Constant, you can actually calculate the number of particles in known quantity of material
Example 3:
How many water molecules are there in 1g of water, H2O? Formula mass of water = (2 x 1) + 16 = 18
Every mole of a substance contains 6 x 1023 particles of ‘it’ (the Avogadro Constant)
Moles water = 1 / 18 = 0
0556
Molecules of water = 0.0556 x 6 x 1023 = 3.34 x 1022
5.Percentage Composition in a Compound:
1. Calculating % Composition (from masses of each element) Divide the mass of each element by the total mass of the compound and multiply by 100
2. Calculating % Composition (from formula)
Calculate formula mass
Divide the total atomic mass of each element by the formula mass and multiply by 100
Example 1
What is the percentage of oxygen in carbon dioxide gas?
mass of oxygen in one mole of carbon dioxide gas = 2×16 g
= 32 g
mass of one mole of carbon dioxide = 12 g + (2×16 g)
= 44 g
percentage of oxygen = (32 g/44 g) × 100
= 72.7 %
Example 2
What is the percentage of water of crystallization in hydrated copper (II) sulphate, CuSO4
Example1
The actual molar mass of the compound in the previous example is 42 g mol-1 What is the molecular formula for this compound?
The molar mass of CH2 = (12+(2×1)) g mol-1= 14 g mol-1
The molecular formula of this compound is (CH2)n, where n is a positive whole number
The value of n = 42 g mol-1 / 14 g mol-1
= 3
The molecular formula is (CH2)3 or more properly C3H6
Examples of where the empirical formula is the same as the molecular formula
Water H2O, methane CH4, propane C3H8 (these molecular formula cannot be ‘simplified’) Examples of where the molecular formula is different from the empirical formula Ethane C2H6 (CH3), phosphorus (V) oxide P4.O10
(P2O5), benzene C6H6 (CH)
Three examples are set out below to illustrate all the situations
The relative atomic masses of the elements (Ar) are given in the tabular format method of solving the problem
Example 2:
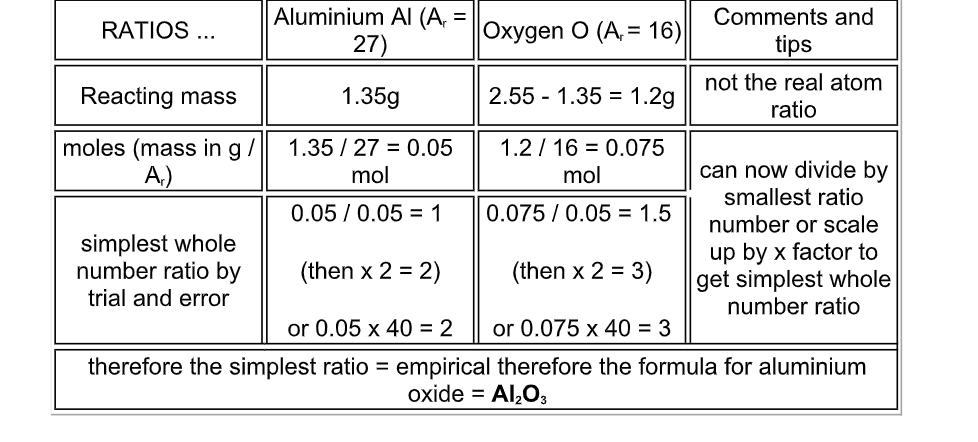
1.35g of aluminium was heated in oxygen until there was no further gain in weight
The white oxide ash formed weighed 2.55g
Deduce the empirical formula of aluminium oxide
Note: to get the mass of oxygen reacting, all you have to do is to subtract the mass of metal from the mass of the oxide formed
Example 3:
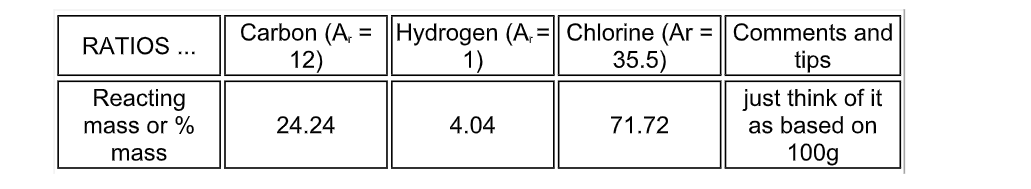
A chlorinated hydrocarbon compound when analysed, consisted of 24
24% carbon, 4
04% hydrogen, 71.72% chlorine
The molecular mass was found to be 99 from another experiment
Deduce the empirical and molecular formula
(you can ‘treat’ the %’s as if they were grams, and it all works out like examples 1 and 2)

8. Reacting Mass Calculations
You can use the ideas of relative atomic, molecular or formula mass and the law of conservation of mass to do quantitative calculations in chemistry
Underneath an equation you can add the appropriate atomic or formula masses
This enables you to see what mass of what, reacts with what mass of other reactants
It also allows you to predict what mass of products are formed (or to predict what is needed to make so much of a particular product)
You must take into account the balancing numbers in the equation (e.g 2Mg), as well of course, the numbers in the formula (e.g O2)
NOTE: The symbol equation must be correctly balanced to get the right answer!
Example 1:
(a) In a copper smelter, how many tonne of carbon (charcoal, coke) is needed to make 16 tonne of copper?
(b) How many tones of copper can be made from 640 tones of copper oxide ore?
(a) 2CuO(s) + C(s) 2Cu(s) + CO2(g) >
(atomic masses Cu=64, O=16, C=12)
Formula Mass ratio is 2 x (64+16) + (12) ==> 2 x (64) + (12 + 2×16)
= Reacting mass ratio 160 + 12 ==> 128 + 44
(In the calculation, impurities are ignored)
12 of C makes 128 of Cu
Scaling down numerically: mass of carbon needed
= 12 x 16 / 128 = 1
5 tonne of C
(b) 160 of CuO make 128 of Cu (or direct from formula 80 CuO 64 Cu)
Scaling up numerically: mass copper formed
= 128 x 640 / 160 = 512 tones Cu
Example 2:
What mass of carbon is required to reduce 20 tonne of iron(II) oxide ore if carbon monoxide is formed in the process as well as iron?
(Atomic masses: Fe = 56, O = 16)
Reaction equation: Fe2O3 + 3C 2Fe + 3CO
Formula mass Fe2O3 = (2×56) + (3×16) = 160
160 mass units of iron oxide reacts with 3 x 12 = 36 mass units of carbon
So the reacting mass ratio is 160: 36
So the ratio to solve is 20: x, scaling down,
x = 36 x 20/160 = 4
5 tones carbon needed
Note: Fe2O3 + 3CO 2Fe + 3CO2 is the other most likely reaction that reduces the iron ore to iron
9 Molar Volume of Gas
Avogadro’s Law states that equal volumes of gases under the same conditions of temperature and pressure contain the same number of molecules
So the volumes have equal moles of separate particles in them
One mole of any gas (or the formula mass in g), at the same temperature and pressure occupies the same volume
This is 24dm3 (24 litres) or 24000 cm3, at room temperature and pressure (r.t.p)
Avogadro’s Law and Molar Volume
Equal volumes of all gases contain the same number of molecules
In this table, N =6
02 X 1023 molecules, while V= 24000 cm3 at room temperature and pressure (r.t.p), or 22400 cm3 at standard temperature and pressure (s.t.p)
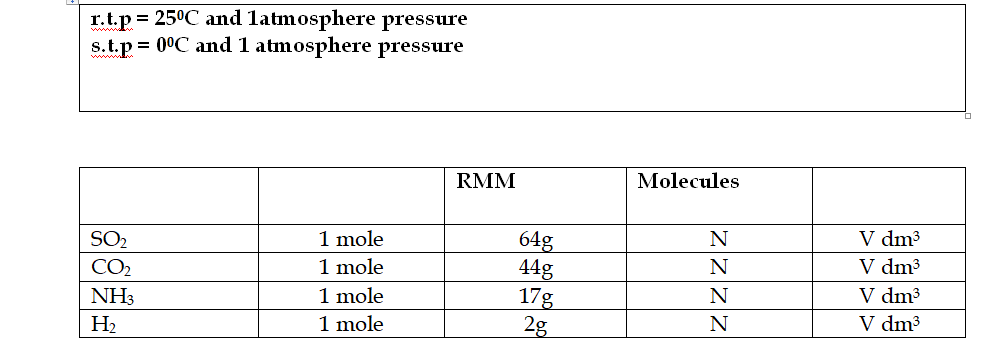
1 mole of any gas always contains the same number of molecules (6.02 X 1023) and hence has the same volume (at the same temperature and pressure)
When gases combine, they do so in small volumes which bear a simple ratio to one another and to the volume of product if gaseous
All volumes must be measured at the same temperature and pressure
Some handy relationships for substance Z below:
moles Z = mass of Z gas (g) / atomic or formula mass of gas Z (g/mol)
mass of Z in g = moles of Z x atomic or formula mass of Z
atomic or formula mass of Z = mass of Z / moles of Z
1 mole = formula mass of Z in g
gas volume of Z = moles of Z x molar volume
moles of Z = gas volume of Z / molar volume
Example 1: What is the volume of 3
5g of hydrogen? [Ar(H) = 1]
Hydrogen exists as H2 molecules, so Mr(H2) = 2, so 1 mole or
Formula mass in g = 2g
So moles of hydrogen = 3
5/2 = 1
75 mol H2 So volume H2 = mol H2 x molar volume = 1
75 x 24 = 42 dm3 (or 42000 cm3)
Example 4:
Given the equation MgCO3(s) + H2SO4(aq) ==> MgSO4(aq) + H2O(l) +CO2(g)
What mass of magnesium carbonate is needed to make 6 dm3 of carbon dioxide at r.t.p?
[Ar’s: Mg = 24, C = 12, O = 16, H =1 and S = 32]
method (a):
Since 1 mole = 24 dm3, 6 dm3 is equal to 6/24 = 0.25 mol of gas From the equation, 1 mole of MgCO3 produces 1 mole of CO2, which occupies a volume of 24 dm3
So 0.25 moles of MgCO3 is need to make 0
25 mol of CO2 Formula mass of MgCO3 = 24 + 12 + 3×16 = 84, So required mass of MgCO3 = mol x formula mass = 0.25 x 84 = 21g
Method (b):
Converting the equation into the required reacting masses
Formula masses: MgCO3 = 84 (from above), CO2 = 12 + 2×16 = 44
MgCO3: CO2 equation ratio is 1 : 1
so 84g of MgCO3 will form 44g of CO2 44g of CO2 will occupy 24dm3 so scaling down, 6 dm3M of CO2 will have a mass of 44 x 8/24 = 11g if 84g MgCO3 ==> 44g of CO2, then
21g MgCO3 ==> 11g of CO2 by solving the ratio, scaling down by factor of 4
10.Reacting Gases
Avogadro’s Law states that ‘equal volumes of gases at the same temperature and pressure contain the same number of molecules’ or moles of gas
This means the molecule ratio of the equation automatically gives us the gas volumes ratio of reactants and products, if all the gas volumes are measured at the same temperature and pressure
This calculation only applies to gaseous reactants or products and if they are all at the same temperature and pressure
Example 1: HCl(g) + NH3(g) NH4Cl(s)
Example 2: N2(g) + 3H2(g) 2NH3(g)
Example 3: C3H8(g) + 5O2(g) CO2(g) + 4H3O(l)
(a) What volume of oxygen is required to burn 25cm3 of propane, C3H8
(b) What volume of carbon dioxide is formed if 5dm3 of propane is burned?
(c) What volume of air (1/5th oxygen) is required to burn propane at the rate of 2dm3 per minute in a gas fire?
Stoichiometric and Ionic Equations
Chemical word equations
For any reaction, what you start with are called the reactants, and what you form are called the products
So any chemical equation shows in some way the overall chemical change of
REACTANTS -> PRODUCTS
This can be written in words or symbols/formulae
The arrow means the direction of change from reactants =to=> products No symbols or numbers are used in word equations
Always try to fit all the words neatly lined up from left to right, especially if it is a long word equation
The word equation is presented to summarise the change of reactants to products
Stoichiometric Equations
Consider the reaction between zinc sulphide and oxygen
The equation can be written as; ZnS(s) + O2(g) ZnO(s) + SO2(g) This is an example of a stoichiometric, or normal chemical(or symbol) equation
From this equation, we can deduce;
Rules on Balancing Symbol equations
1. You should know what the reactants and products are,
2. write a word equation with appropriate reactants on the left and products on the right
3. Writing the correct symbol or formula for each equation component
Numbers in a formula are written as subscripts after the element concerned
NOTE: If the number is 1 itself, by convention, no number is shown in a formula or before a formula
4 Using numbers if necessary to balance the equation
5 If all is correct, then the sum of atoms for each element should be the same on both side of the equation arrow
a) in other words: atoms of products = atoms of reactants
This is a chemical conservation law of atoms and later it may be described as the ‘law of conservation of mass
b) the equations are first presented in ‘picture’ style and then written out fully with state symbols
c) The individual formulas involved and the word equations have already been presented above
Ionic Equations
1. Acid-base reactions:
Acids can be defined as proton donors
A base can be defined as a proton acceptor
Any acid-alkali neutralisation involves the hydroxide ion is (base) and this accepts a proton from an acid
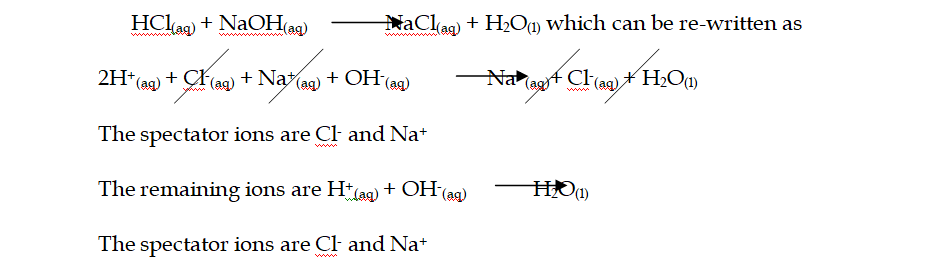
2. Insoluble salt formation:
An insoluble salt is made by mixing two solutions of soluble compounds to form the insoluble compound in a process called ‘precipitation’
A precipitation reaction is generally defined as ‘the formation of an insoluble solid on mixing two solutions or a gas bubbled into a solution’
(a) Silver chloride is made by mixing solutions of solutions of silver nitrate and sodium chloride

–(aq) -> CaCO3(s)
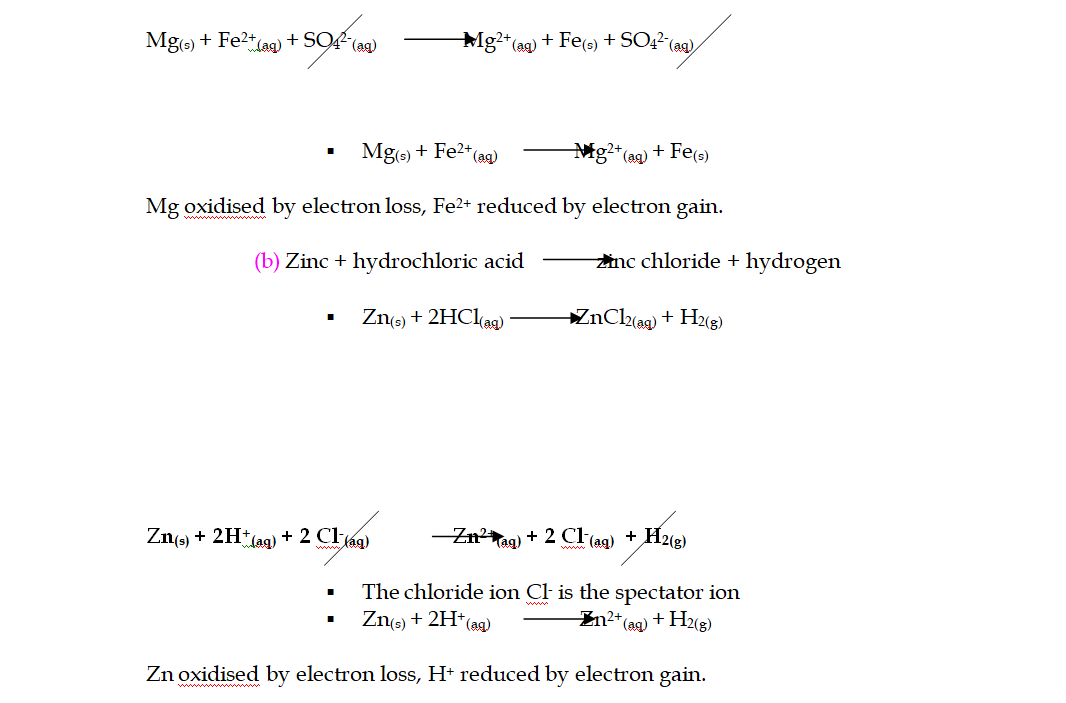
(3) Redox reaction analysis:
(a) Magnesium + iron (II) sulphate magnesium sulphate + iron
No electrons show up in the full equations because electrons lost by x = electrons gained by y!!
11. MOLARITY
It is very useful to be known exactly how much of a dissolved substance is present in a solution of particular concentration or volume of a solution
So we need a standard way of comparing the concentrations of solutions
The concentration of an aqueous solution is usually expressed in terms of moles of dissolved substance per cubic decimetre, mol dm-3 , this is called molarity, sometimes denoted in shorthand as M
Note: 1dm3 = 1 litre = 1000ml = 1000 cm3 , so dividing cm3 /1000 gives dm3 , which is handy to know since most volumetric laboratory apparatus is calibrated in cm3 (or ml), but solution concentrations are usually quoted in molarity, that is mol/dm3 (mol/litre)
Equal volumes of solution of the same molar concentration contain the same number of moles of solute i.e the same number of particles as given by the chemical formula
You need to be able to calculate:
You should recall and be able to use each of the following relationships
(1) molarity of Z = moles of Z / volume in dm3 (2) molarity x formula mass of solute = solute concentration in g/dm3 , dividing this by 1000 gives the concentration in g/cm3 (3) (concentration in g/dm3 ) / formula mass = molarity in mol/dm3 , (4) moles Z = mass Z / formula mass of Z
(5) 1 mole = formula mass in grams
Example 1
What mass of sodium hydroxide (NaOH) is needed to make up 500 cm3 (0.5 dm3 ) of a 0.5M solution?
[Ar’s: Na = 23, O = 16, H = 1]
1 mole of NaOH = 23 + 16 + 1 = 40g
for 1000 cm3 (1 dm3 ) of 0.5M you would need 0.5 moles NaOH
which is 0.5 x 40 = 20g however only 500 cm3 of solution is needed compared to 1000 cm3 so scaling down: mass NaOH required = 20 x 500/1000 = 10g
Example 2
How many moles of H2SO4 are there in 250cm3 of a 0.8M sulphuric acid solution? What mass of acid is in this solution?
[Ar’s: H = 1, S = 32, O = 16]
formula mass of sulphuric acid = 2 + 32 + (4×16) = 98, so 1 mole = 98g if there was 1000 cm3 of the solution, there would be 0.8 moles H2SO4 but there is only 250cm3 of solution, so scaling down
moles H2SO4 = 0,8 x (250/1000) = 0.2 mol mass = moles x formula mass, which is 0
2 x 98 = 19.6g of H2SO4
Example 3
5.95g of potassium bromide was dissolved in 400cm3 of water [Ar’s: K = 39, Br = 80]
moles = mass / formula mass, (KBr = 39 + 80 = 119)
mol KBr = 5.95/119 = 0.05 mol
400 cm3 = 400/1000 = 0.4 dm3
molarity = moles of solute / volume of solution molarity of KBr solution = 0.05/0.4 = 0.125M
Example 4
What is the concentration of sodium chloride (NaCl) in g/dm3 and g/cm3 in a 1.50 molar solution?
At Masses: Na = 23, Cl = 35.5, formula mass NaCl = 23 + 35.5 = 58.5 Therefore concentration = 1.5 x 58.5 = 87.8 g/dm3 , and concentration = 87.75 / 1000 = 0.0878 g/cm3
Example 5
A solution of calcium sulphate (CaSO4) contained 0.5g dissolved in 2dm3 of water
Calculate the concentration in (a) g/dm3 ,
(b) g/cm3 and
(c) mol/dm3
(a) concentration = 0.5/2 = 0.25 g/dm3 , then since 1dm3 = 1000 cm3
(b) concentration = 0.25/1000 = 0
00025 g/cm3 (c) At.masses: Ca = 40, S = 32, O = 64, f
mass CaSO4 = 40 + 32 + (4 x 16) = 136 moles CaSO4 = 0.5 / 136 = 0.00368 mol
concentration CaSO4 = 0.00368 / 2 = 0.00184 mol/dm3
12. Titration: Acid and Alkali
Titrations can be used to find the concentration of an acid or alkali from the relative volumes used and the concentration of one of the two reactants
You should be able to carry out calculations involving neutralisation reactions in aqueous solution given the balanced equation or from your own practical results
1. Note again: 1dm3 = 1 litre = 1000ml = 1000 cm3 , so dividing cm3 /1000 gives dm3
2. and other useful formulae or relationships are:
In most volumetric calculations of this type, you first calculate the known moles of one reactant from a volume and molarity
Then, from the equation, you relate this to the number of moles of the other reactant, and then with the volume of the unknown concentration, you work out its molarity
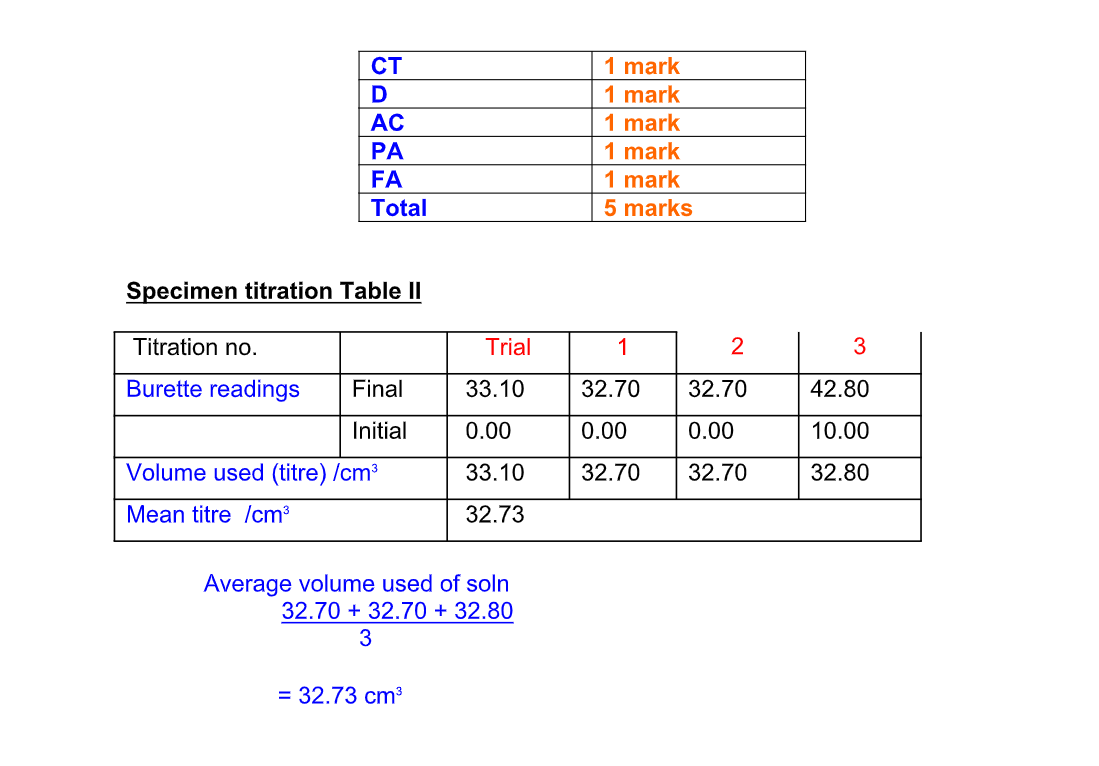
Example 1:
25 cm3 of a sodium hydroxide solution was pipetted into a conical flask and titrated with 0
2M hydrochloric acid
Using a suitable indicator it was found that 15 cm3 of acid was required to neutralise the alkali
Calculate the molarity of the sodium hydroxide and concentration in g/dm3
equation NaOH(aq) + HCl(aq) NaCl(aq) + H2O(l)
moles HCl = (15/1000) x 0.2 = 0.003 mol
moles HCl = moles NaOH (1 : 1 in equation)
so there is 0.003 mol NaOH in 25 cm3 scaling up to 1000 cm3 (1 dm3 ), there are
0.003 x (1000/25) = 0.12 mol NaOH in 1 dm3 molarity of NaOH is 0.12M or mol dm-3 since mass = moles x formula mass, and Mr(NaOH) = 23 + 16 + 1 = 40
concentration in g/dm3 is 0
12 x 40 = 4.41g/dm3
Example 2:
20 cm3 of a sulphuric acid solution was titrated with 0.05M potassium hydroxide
If the acid required 36 cm3 of the alkali KOH for neutralisation what was the concentration of the acid?
equation 2KOH(aq) + H2SO4(aq) K2SO4 + 2H2O(l) mol KOH = 0.05 x (36/1000) = 0
0018 mol mol H2SO4 = mol KOH / 2 (because of 1 : 2 ratio in equation above) mol H2SO4 = 0.0018/2 = 0.0009 (in 20 cm3 ) scaling up to 1000 cm3 of solution = 0
0009 x (1000/20) = 0.045 mol mol H2SO4 in 1 dm3 = 0
045, so molarity of H2SO4 = 0.045M or mol dm-3 since mass = moles x formula mass, and Mr(H2SO4) = 2 + 32 + (4×16) = 98 Concentration in g/dm3 is 0
045 x 98 = 4.41g/dm3 How to carry out a titration
The diagrams show the typical apparatus (1)-(6) used in manipulating liquids and on the left a brief three stage description of titrating an acid with an alkali:

Volumetric Analysis (Titrations)
A titration is a laboratory procedure where a measured volume of one solution is added to a known volume of another reagent until the reaction is complete
The operation is an example of volumetric (titrimetric) analysis
The equivalence point is usually shown by the colour change of an indicator and is known as the end-point
Volumetric analysis is a powerful technique, which is used in a variety of ways by chemists in many different fields
Practical Aspects
The practical aspects of titrations are required in the assessment of practical skills
Knowledge of the techniques of titrations is expected but it would be normal to assume that all apparatus would have been washed with distilled/deionised water
The description should include which reagent is placed in the burette, name of indicator (but no reason for choice of indicator), detection of endpoint and what should be observed, and repetition for accuracy
When you have finished this section you should be able to:
Use of a Volumetric Flask
To prepare a solution of precisely known concentration (a standard solution), a definite amount of solute must be dissolved in a solvent to give a definite volume of solution
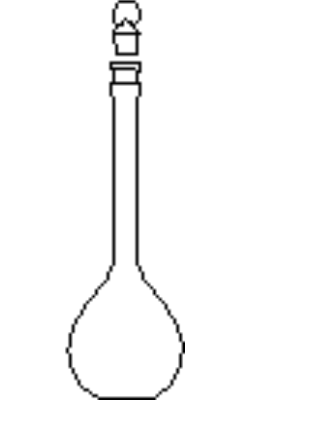
The definite amount of material is measured by weighing, and the definite volume of solution prepared in a volumetric flask
A volumetric flask contains a definite volume when correctly filled to the calibration mark at the temperature stated on the flask
Tip the solid from a weighing bottle into a large (250 cm3) beaker and add about 50 cm3 of distilled water from a wash bottle
Stir well with a glass rod to dissolve
Take great care not to lose any of the solution and remember to wash the solution off the stirring rod back into the beaker
Rinse out the volumetric flask with distilled water and pour the cold solution into the flask through a clean filter funnel
Wash out the beaker several times and add all the washings to the flask
Now fill the flask to within about 1 cm of the calibration mark on the neck
Finally add water dropwise until the meniscus just rests on the calibration mark
Stopper the flask and invert a number of times to thoroughly mix the contents
Use of a Pipette The pipette is designed to deliver a definite fixed volume of liquid when correctly filled to its calibration mark
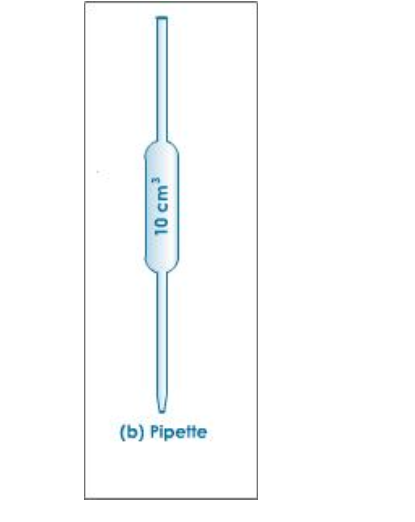
Before use, a pipette must be washed out with the solution it is to measure
To fill the pipette, use a safety filler to suck solution up a few centimetres above the calibration mark
Let the solution down until the bottom of the meniscus just touches the calibration mark
For a titration the contents of the pipette are run into a conical flask, which has been well washed with distilled water
Allow the pipette to drain for about 20 seconds, then touch the tip to the surface of the liquid in the conical flask
The volume of solution delivered by the pipette is known as the aliquot
Use of a Burette
The burette is designed to deliver definite but variable volumes of liquid
First rinse out the burette with the solution it is to contain
Clamp the burette vertically in a stand
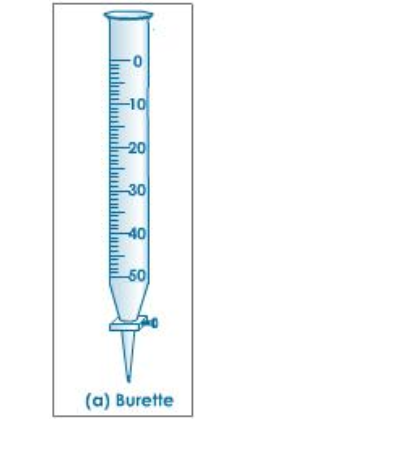
Fill the burette carefully using a beaker and a filter funnel
Open the tap briefly to fill the burette below the tap making sure there are no trapped air bubbles
Read the burette scale by observing the position of the bottom of the liquid meniscus, making sure your eyes are level with the graduation mark
To observe the meniscus more clearly, hold a white card behind the burette
Record the volume reading to the nearest 0.05 cm3
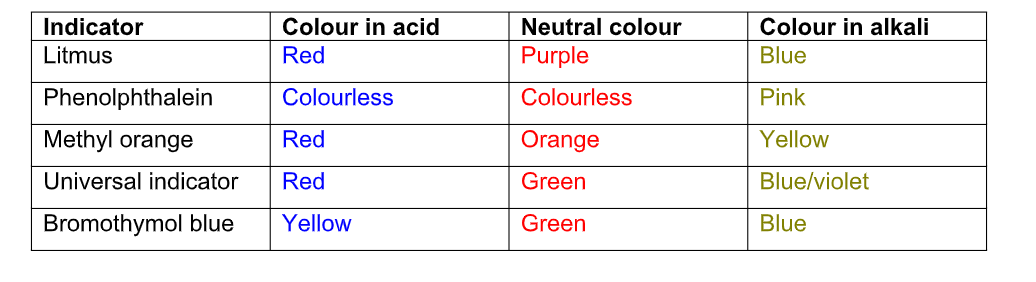
Some common indicators
Titration Technique
When performing a titration, place the conical flask containing the aliquot on a white tile under the burette so that the tip of the burette is inside the mouth of the conical flask to avoid splashing
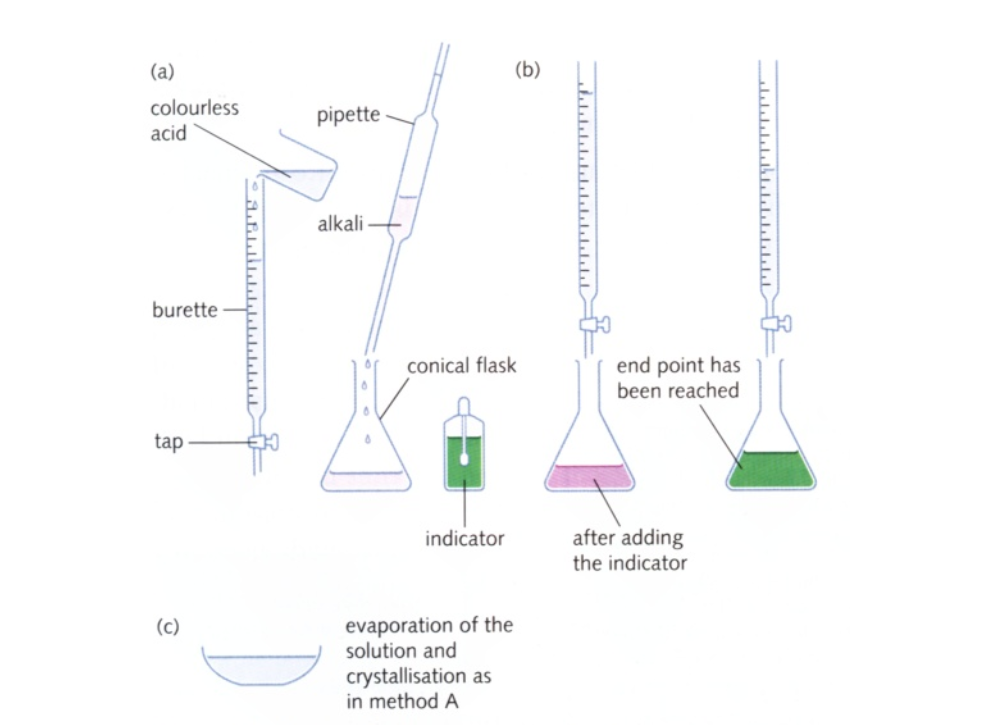
Add a few drops of a suitable indicator to the solution in the conical flask
First perform a ‘rough’ titration by taking the burette reading and running in the solution in approximately 1 cm3 portions, while swirling the flask vigorously
When the end-point is reached, as shown by the indicator changing colour, quickly close the tap
The new burette reading will give you a rough idea (to within about 1 cm3) of the volume to be added
Now repeat the titration with a fresh aliquot
As the rough end-point volume is approached, add solution from the burette one drop at a time until the indicator changes colour
Record the volume
The volume run out from the burette to reach the end point is known as the titre
Recording Titration Results
The results of a titration should be recorded;
Record the titration results in the form of a table

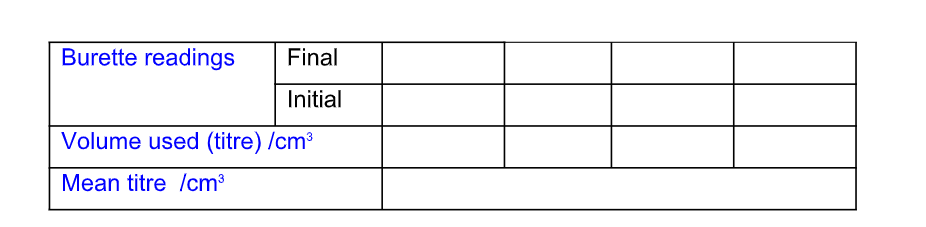
Accuracy
Record burette readings to the nearest 0.05 cm3 (approximately 1 drop)
Consecutive titrations should agree to within 0.10 cm3 and, strictly, you should repeat the titrations until this is achieved
However you may not have either the time or materials available to do this
With practice, your technique should improve so that you should not need to do more than 4 titrations (1 trial + 3 accurate)
Calculate and use the mean (average) of the two (or preferably three) closest consecutive readings and quote this to the nearest 0.05 cm3
What do examiners look for in your answer sheet?
Calculating the Concentration of a Solution from Titration Data
When you have finished this section you should be able to:
14.0.0 Organic Chemistry 1
Products from Oil
Coal, Oil and Natural Gas Formation – Fossil Fuels
Just as coal has formed by the action of heat and pressure on the remains of trees and plants on land over millions of years, so oil and natural gas have formed by the action of heat and pressure on the remains of sea plants and animals over millions of years
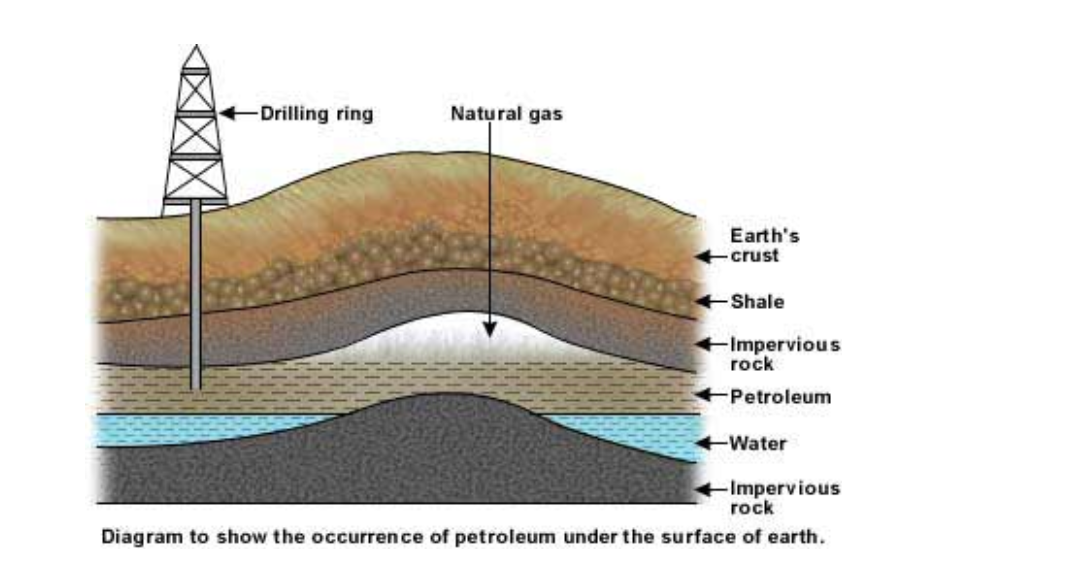
The remains were buried in sediments which excluded the air (kept out oxygen) and stopped them decaying
More sediment buried the remains deeper and deeper until pressure and heat eventually turned them into coal, oil and natural gas
They are called fossil fuels because they are buried underground (from Latin fossilis – dug up)
Fossil fuels are a finite resource and non-renewable
The oil deposits are formed in porous rock sediments
Porous rock has pores in it
Pores are small holes (see for example sandstone)
The small holes allow the oil and natural gas to pass through the rock and rise until they are stopped by a layer of non-porous rock
Non-porous rock (for example shale) has no holes, and acts as a barrier to prevent the oil and natural gas rising
The oil and natural gas become trapped underground
The oil is called crude oil (or petroleum, from Latin – rock oil), and has natural gas in it or in a pocket above it trapped by non-porous rock
Drilling through the rock allows the oil and gas to escape to the surface
Natural gas is mostly methane (CH4)
Crude oil is a mixture of substances (mostly hydrocarbons)
So what are Hydrocarbons?
Crude oil is a mixture of substances which are mostly hydrocarbons
A hydrocarbon is a compound containing hydrogen and carbon only
Since crude oil is a mixture of different hydrocarbon compounds, the different hydrocarbons will have different boiling points
A sample of crude oil will therefore have a range of boiling points, and the mixture can be separated by fractional distillation
Fractional Distillation of Crude Oil

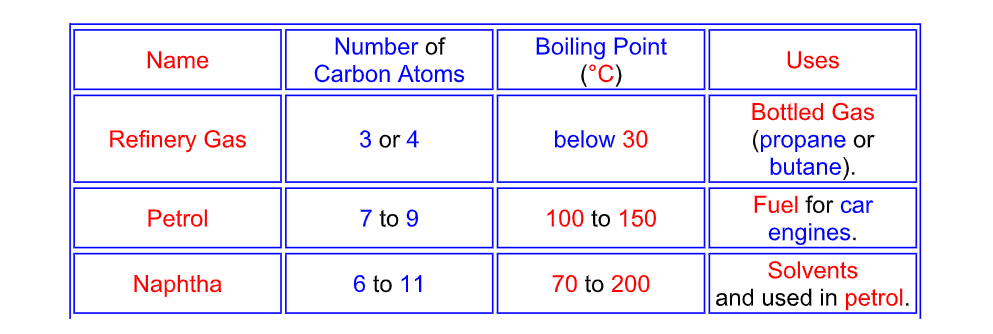
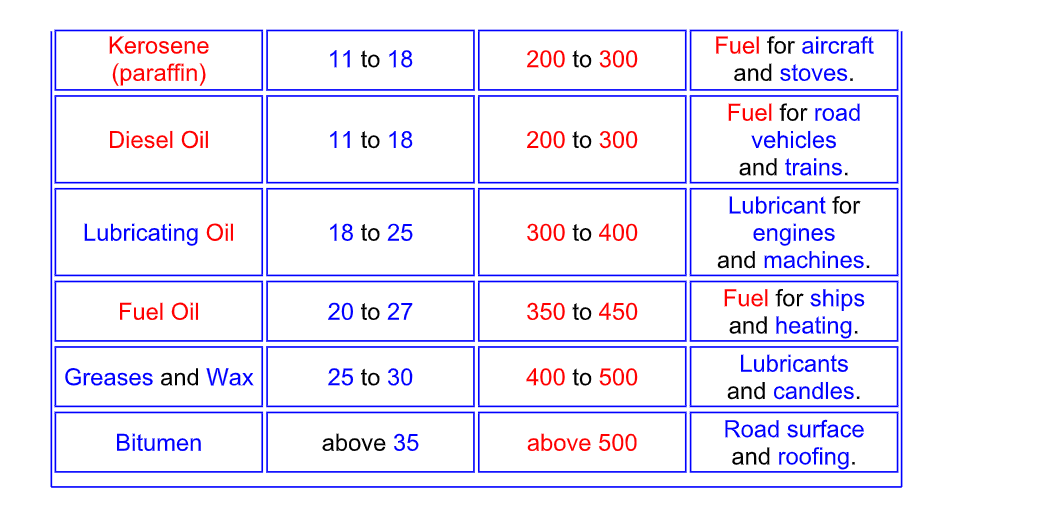
Naming the fractions
The hydrocarbon fractions are mainly alkanes
Crude oil is heated until it boils and then the hydrocarbon gases are entered into the bottom of the fractionating column
As the gases go up the column the temperature decreases
The hydrocarbon gases condense back into liquids and the fractions are removed from the sides of the column
The smaller the hydrocarbon molecule, the further it rises up the column before condensing
The fractionating column operates continuously
The temperatures shown are approximate
A sample of crude oil may be separated in the laboratory by fractional distillation
The collection vessel is changed as the temperature rises to collect the different fractions
Naming hydrocarbons
Hydrocarbons are named according to the number of carbon atoms in the molecule
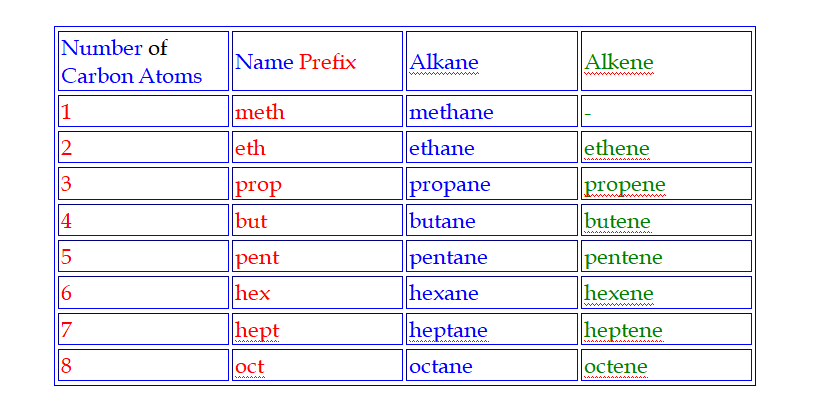
Meth is pronounced meeth (like teeth), Eth is pronounced eeth (like teeth), Prop is pronounced prope (like rope), But is pronounced bute (like beauty)
Pent is pronounced pent (like pentagon)
Hex is pronounced hex (like hexagon)
Hept is pronounced hept (like heptagon)
Oct is pronounced oct (like octagon)
The hydrocarbon fractions are mainly alkanes
Properties of Different Fractions
The different hydrocarbon fractions obtained from crude oil condense at different temperatures
The larger the hydrocarbon molecule (the more carbon atoms it has)
1) The higher the condensing temperature (the higher the boiling point)
2) The more viscous it is (it takes longer to flow – like syrup)
3) The less volatile it is (it evaporates less quickly)
4) The less flammable it is (it does not set fire so easily)
Gases from volatile hydrocarbons are denser than air and pose a fire hazard at ground level
This is why ignition sources (such as smoking) are not allowed at petrol stations
Families of organic compounds
Homologous series
Organic compounds belong to different families, though all are based on carbon C, hydrogen H, and other elements such as oxygen O and nitrogen N etc The compounds in each family have a similar chemical structure and a similar chemical formula
Each family of organic compounds forms what is called a homologous series
Different families arise because carbon atoms readily join together in chains (catenation) and strongly bond with other atoms such as hydrogen, oxygen and nitrogen
The result is a huge variety of ‘organic compounds’
The name comes from the fact that most of the original organic compounds studied by chemists came from plants or animals
A homologous series is a family of compounds which have a general formula and have similar chemical properties because they have the same functional group of atoms (e.g C=C alkene, C-OH alcohol or -COOH carboxylic acid)
Members of a homologous series have similar physical properties such as appearance, melting/boiling points, solubility etc but show trends in them e.g steady increase in melting/boiling point with increase in carbon number or molecular mass
The molecular formula represents a summary of all the atoms in the molecule
The structural or displayed formula shows the full structure of the molecule with all the individual bonds and atoms shown (though there are different ‘sub-styles’ of varying detail, see below)
What are alkanes?
These are obtained directly from crude oil by fractional distillation
They are saturated hydrocarbons and they form an homologous series called alkanes with a general formula CnH2n+2 Saturated hydrocarbons have no C=C double bonds, only carbon-carbon single bonds, and so has combined with the maximum number of hydrogen atoms
i.e no more atoms can add to it
Alkanes are the first homologous series
Examples of alkanes are:
The gases Methane CH4, ethane C2H6, propane C3H8, butane C4H10
Liquids Pentane C5H12, hexane C6H14 C7H16 etc
The Names of all alkanes end in …ane
Names
Alkanes are the simplest homologous series of compounds and their names follow this pattern,
CH4 – methane
C2H6 – ethane
C3H8 – propane
C4H10 – butane
C5H12 – pentane
I.e they have a prefix (meth-, eth-, prop-, but-, etc), which depends on the number of carbon atoms in the molecule and a common suffix (-ane)
The general chemical form la for an alkane is CnH2n+2
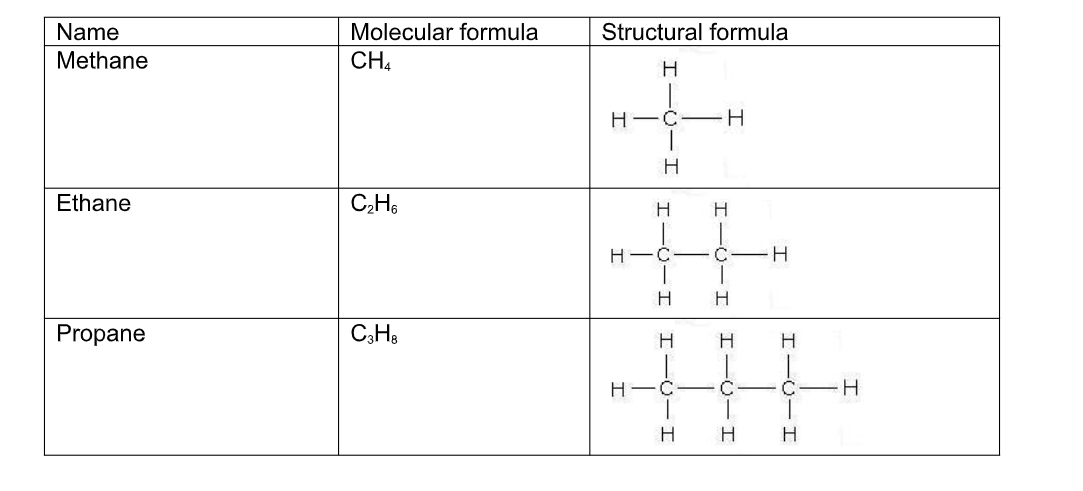
Structural formulae
As well as using a normal type of molecular formula to describe an organic molecule, they can be represented by drawing out their structure i.e by showing how the atoms are connected, or bonded to each other
In order to do this a few rules have to be followed;
(i) Carbon atoms must be bonded four times
(iii) Hydrogen atoms must bond only once
Name Molecular formula Structural formula
Isomerism
Isomerism occurs when two or more compounds have the same chemical formula but have different structures, e.g for the molecular formula C4H10 there are two possibilities – one ‘linear’ and one with carbon chain ‘branching’
Butane is linear while its branched isomer is methyl propane
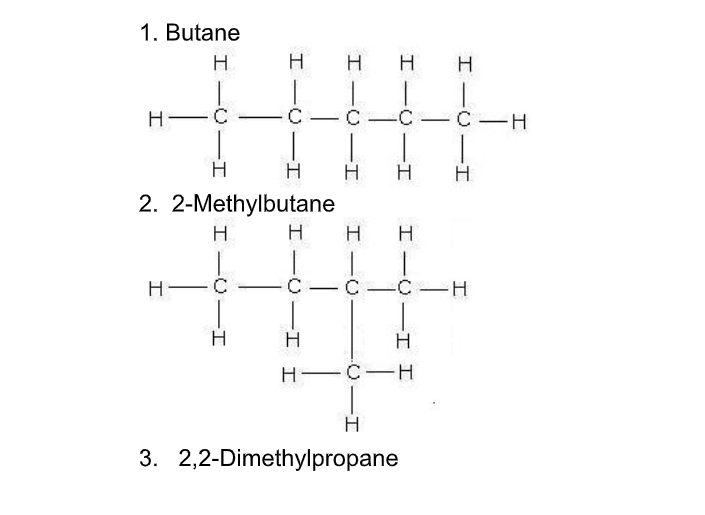
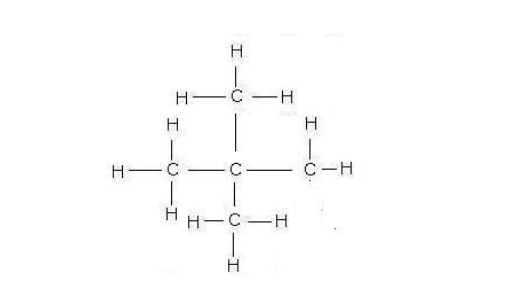
As the number of carbon atoms increases, the number of possible isomers increases rapidly
All families or homologous series exhibit isomerism
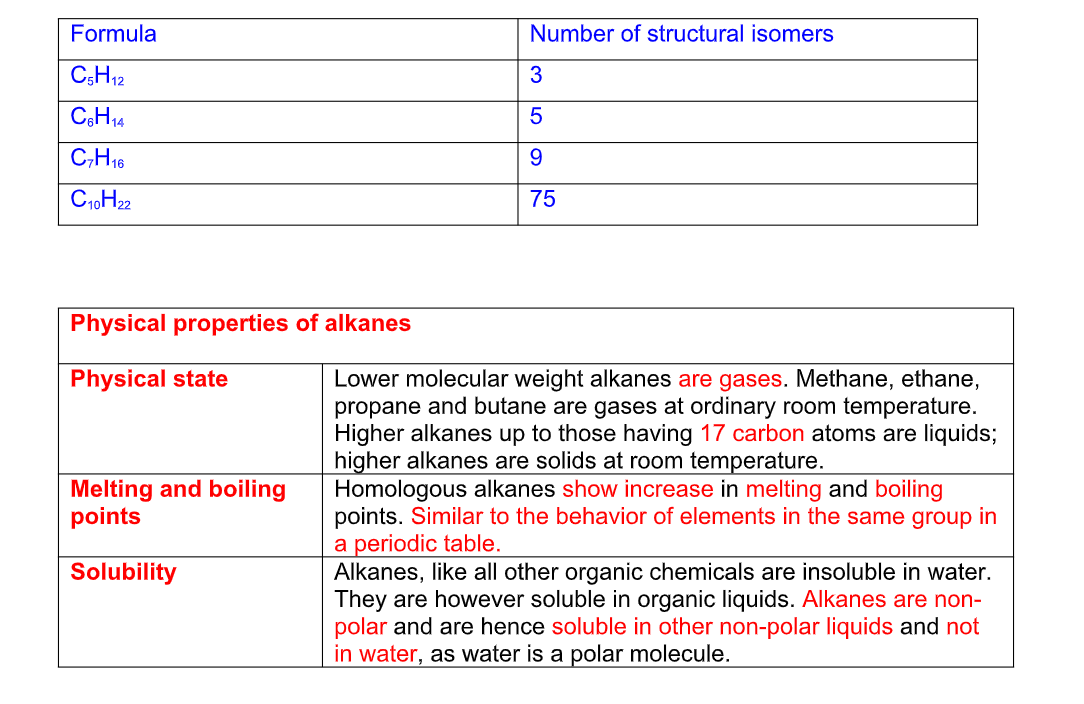
Physical properties of alkanes
Physical state Lower molecular weight alkanes are gases
Methane, ethane, propane and butane are gases at ordinary room temperature
Higher alkanes up to those having 17 carbon atoms are liquids; higher alkanes are solids at room temperature
Melting and boiling points Homologous alkanes show increase in melting and boiling points
Similar to the behavior of elements in the same group in a periodic table
Solubility Alkanes, like all other organic chemicals are insoluble in water
They are however soluble in organic liquids
Alkanes are non-polar and are hence soluble in other non-polar liquids and not in water, as water is a polar molecule
Chemical Reactions of Alkanes
1.Substitutional reactions of alkanes
Alkanes are most inert of all homologous series
They are not very reactive unless burned
But they will react with strong oxidising chemicals like chlorine when heated or subjected to u.v light
A substitution reaction occurs and a chloro-alkane is formed e.g a hydrogen atom is swapped for a chlorine atom and the hydrogen combines with a chlorine atom forming hydrogen chloride
This process is called halogenation
The UV light causes the formation of free radical halogen atoms by providing enough energy for the bond between the two halogen atoms to break
A halogen atom attacks the alkane, substituting itself for a hydrogen atom
This substitution may occur many times in an alkane before the reaction is finished
2. Combustion
Alkanes, along with all other types of hydrocarbon, will burn in an excess of oxygen to give carbon dioxide and water only as the products,
e.g CH4 (g) + 2O2(g) CO2(g) + 2H2O(g) in general,
CnH2n+2(g) + (1.5n+0.5)O2(g) nCO2(g) + (n+1)H2O(g)
If there is not enough oxygen present then instead of carbon dioxide, carbon monoxide, CO, is produced
Carbon monoxide is particularly toxic and absorbed into blood, through respiration, very easily
For domestic heating systems it is particularly important that enough air can get to the flame to avoid carbon monoxide being generated in the home
Car engines also require a lot of air and there is a lot of research going on to make the internal combustion engine more efficient, and so put out less carbon monoxide
3. Reactivity
Alkanes are saturated hydrocarbons
Molecules of saturated hydrocarbons contain only single bonds between all carbon atoms in the series
Hence their reactivity with other chemicals is relatively low
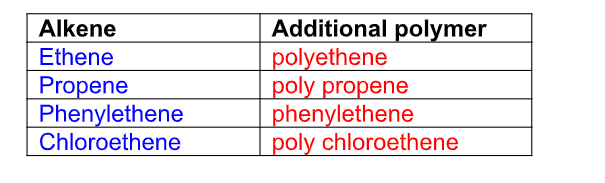
What are alkenes?
Hydrocarbons, which contain two hydrogen atoms less than the corresponding alkanes, are called alkenes
They have one double bond and are unsaturated carbon compounds
Alkenes cannot be obtained directly from crude oil
They can only be obtained by cracking of alkanes
Cracking
In industry the fractions obtained from the fractional distillation of crude oil are heated at high pressure in the presence of a catalyst to produce shorter chain alkanes and alkenes
E.g C10H22 C5H12 + C5H10
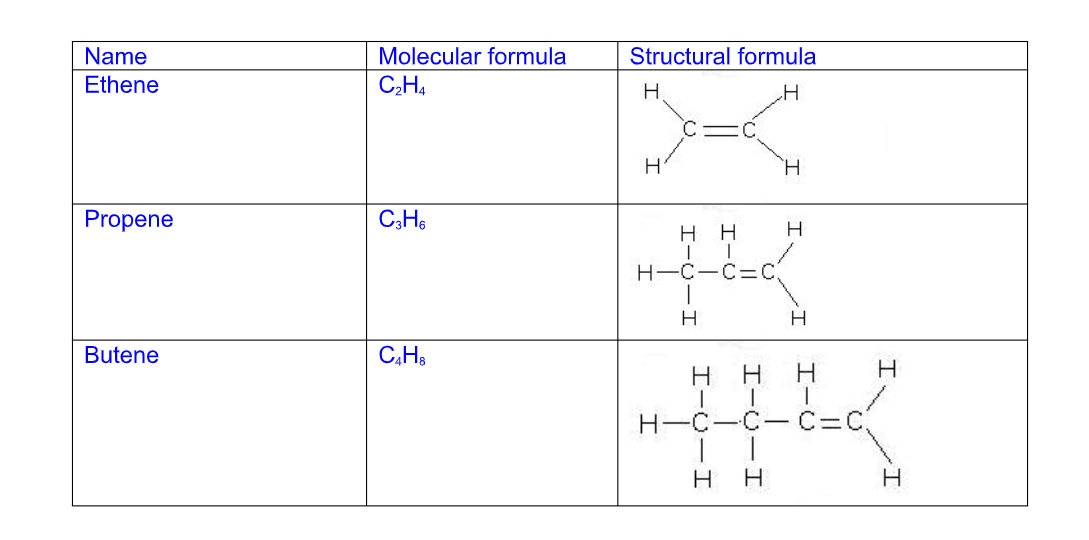
They are unsaturated hydrocarbons with a general formula CnH2n
Unsaturated means the molecule has a C=C double bond to which atoms or groups can add after breaking the double bond
Alkenes all have a C=C double bond in their structure and their names follow this pattern
Their names end i.e ….ene
C2H4 – ethene
C3H6 – propene
C4H8 – butene
C5H10 – pentene
The general chemical formula for an alkene is CnH2n
The general chemical formula for an alkene is CnH2n
(2) Addition reactions of alkenes :
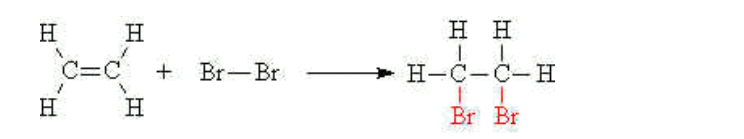
(i) Bromination
The double bond of an alkene will undergo an addition reaction with aqueous bromine to give a dibromo compound
The orange bromine water is decolourised in the process
E.g ethene reacts with bromine water to give 1,2-dibromoethane,
(ii) Hydrogenation
Alkenes may be turned into alkanes by reacting the alkene with hydrogen gas at a high temperature and high pressure
A nickel catalyst is also needed to accomplish this addition reaction
E.g ethene reacts with hydrogen to give ethane,
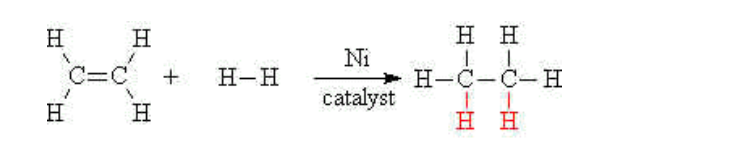
This reaction is also called saturation of the double bond
In ethene the carbon atoms are said to be unsaturated
In ethane the carbon atoms have the maximum number of hydrogen atoms bonded to them, and are said to be saturated
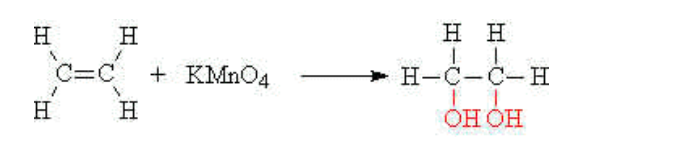
(iii) Oxidation
The carbon-carbon double bond may also be oxidised i.e have oxygen added to it
This is accomplished by using acidified potassium manganate (VII) solution at room temperature and pressure. The purple manganate (VII) solution is decolourised during the reaction
E.g ethene reacts with acidified potassium manganate (VII)(aq) to give ethan-1,2-diol,
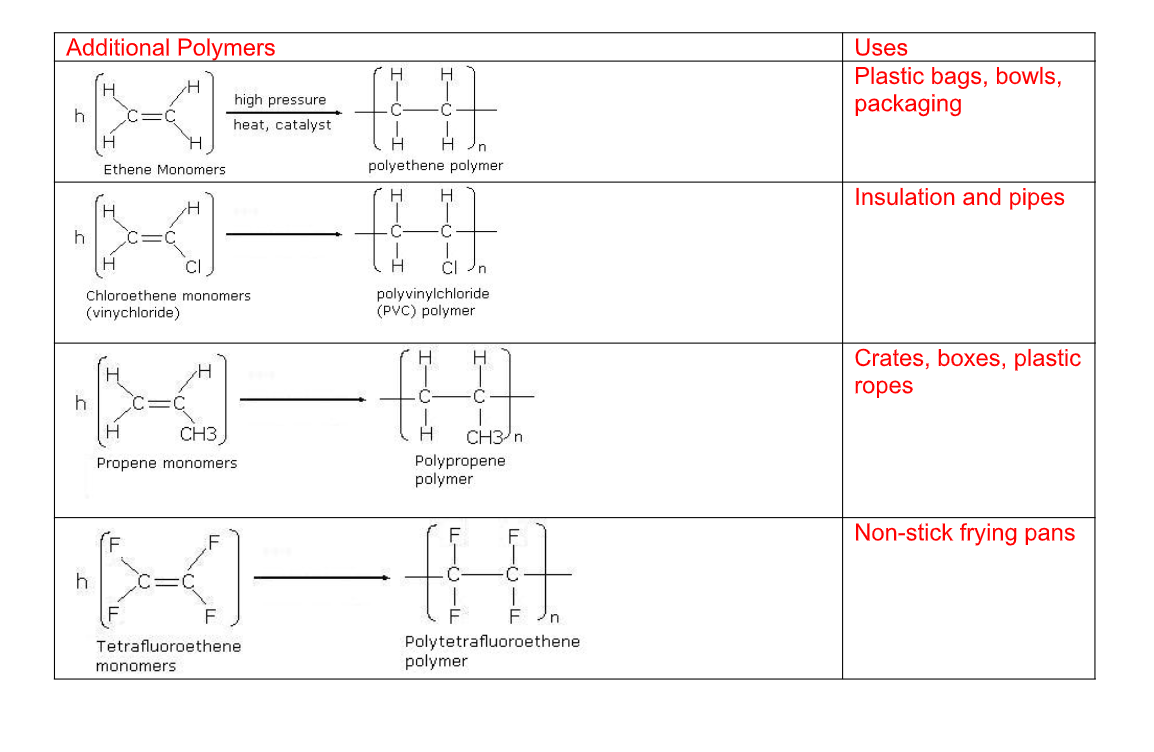
(3) Addition polymerisation :
All alkenes will react with free radical initiators to form polymers by a free radical addition reaction
Some definitions
monomer – a single unit e.g an alkene
The alkene monomer has the general formula:
Where R is any group of atoms, e.g R=CH3 for propene
The reaction progresses by the separate units joining up to form giant, long chains
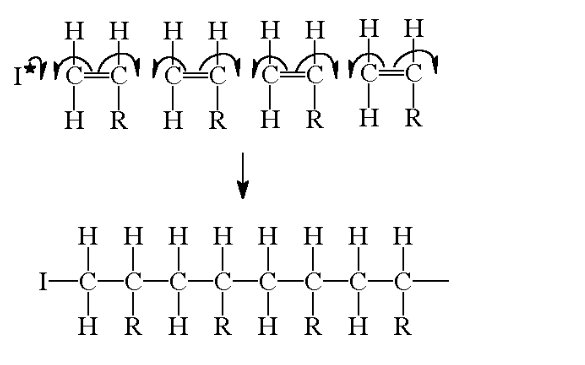
Polymer– a material produced from many separate single monomer units joined up together
An addition polymer is simply named after the monomer alkene that it is prepared from
The structure above shows just 4 separate monomer units joined together
In a real polymer, however, there could be 1000’s of units joined up to form the chains
This would be extremely difficult to draw out and so the structure is often shortened to a repeat unit
There are 3 stages to think about when drawing a repeat unit for a polymer
1) Draw the structure of the desired monomer
2) Change the double bond into a single bond and draw bonds going left and right from the carbon atoms
3) Place large brackets around the structure and a subscript n and there is the repeat unit
Laboratory preparation of ethene gas
In the lab ethene is prepared by cracking kerosene or candle wax
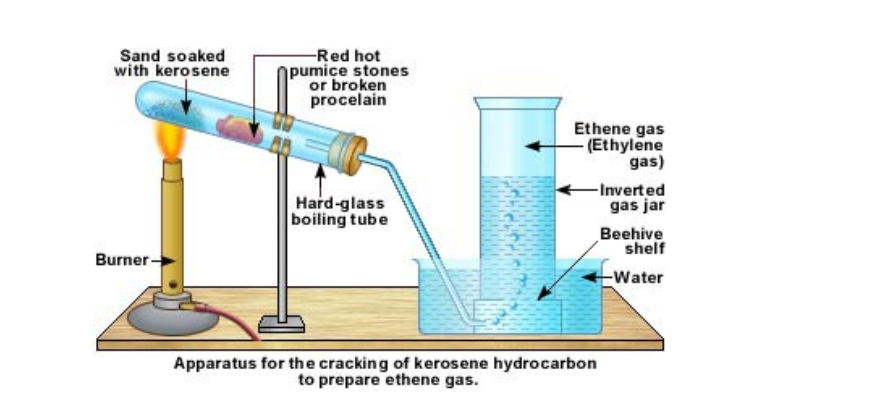
Kerosene is poured over sand and this is kept at the bottom of a hard glass test tube
A few pieces of pumice stone or porcelain is kept a little distance away
The sand is slowly heated
After a while the porcelain portion of the test tube is heated
This is done alternately
The heated kerosene first vaporizes and then cracks
When the vapours pass over the hot porcelain, they crack again into smaller and smaller molecules
The gases are then passed over water
Ethene is collected by downward displacement of water
It can be understood that this method for collecting ethene gas does not give pure ethene gas
This is because from cracking, we get many types of molecules
All those, which are lighter than water and insoluble in water, will be collected
Ethene by dehydration of alcohols
To obtain pure ethene gas, another method is followed
This is from a chemical reaction with ethanol and concentrated sulphuric acid
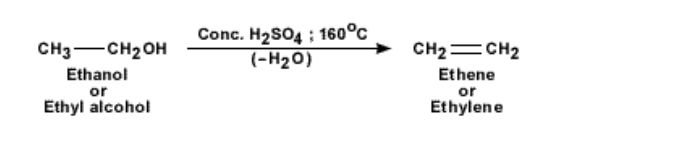
The temperature of the mixture of ethanol or ethyl alcohol and concentrated sulphuric acid is increased to 160°C
The acid acts as a dehydrating agent and picks up a water molecule from the ethanol molecule, leaving the reaction product as ethene gas
The laboratory equipment to produce ethene gas is shown below
About 20 to 25 ml of ethanol is taken in a round bottomed flask
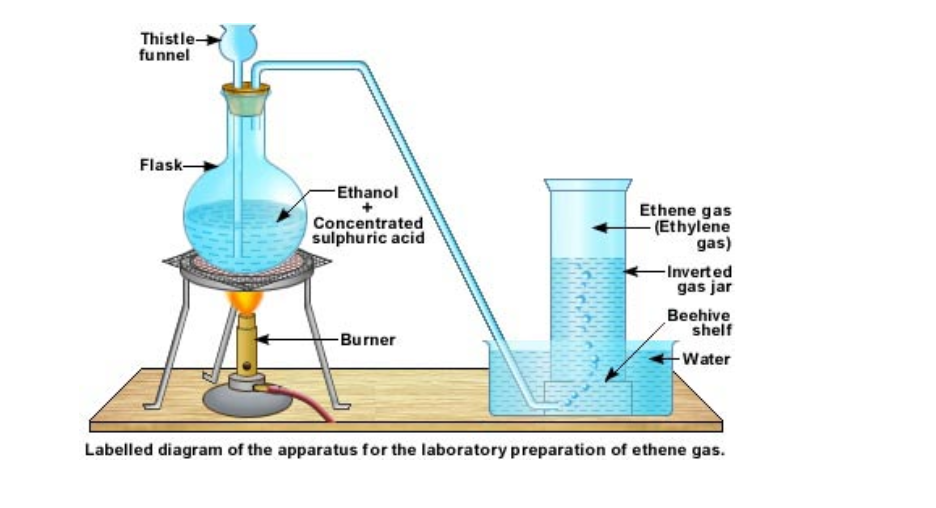
Concentrated sulphuric acid is added to it from a thistle funnel slowly
Heat is supplied from a Bunsen burner and the temperature of the flask is raised to 160°C
Ethene gas starts evolving and it can be collected over water by downward displacement of water
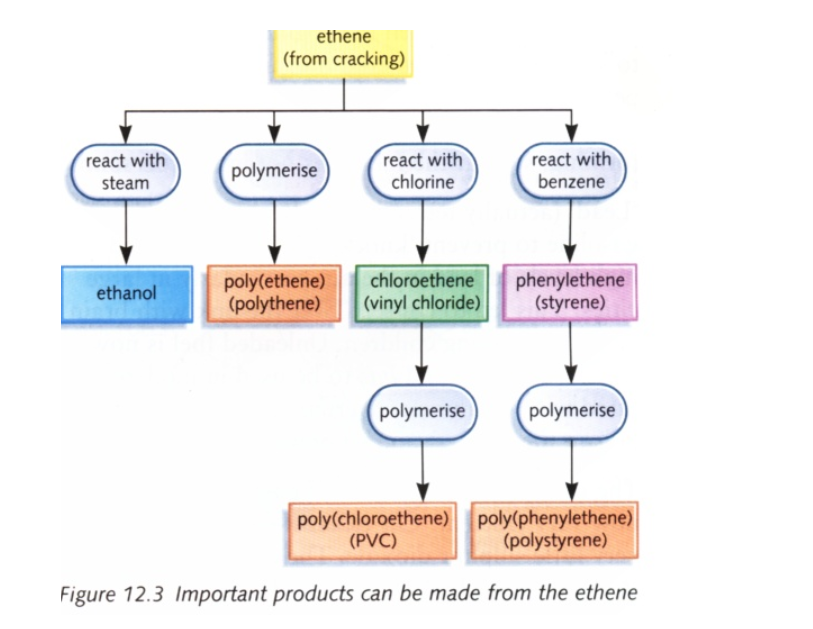
Uses of ethene
Ethylene glycol is used for making artificial fibbers like polyesters
These plastics are made from polymerization of ethene into polythene
Polythenes are used for making bags, electrical insulation, etc
Ethene is used artificial ripening of fruits such as mangoes, bananas, etc
What are Alkynes?
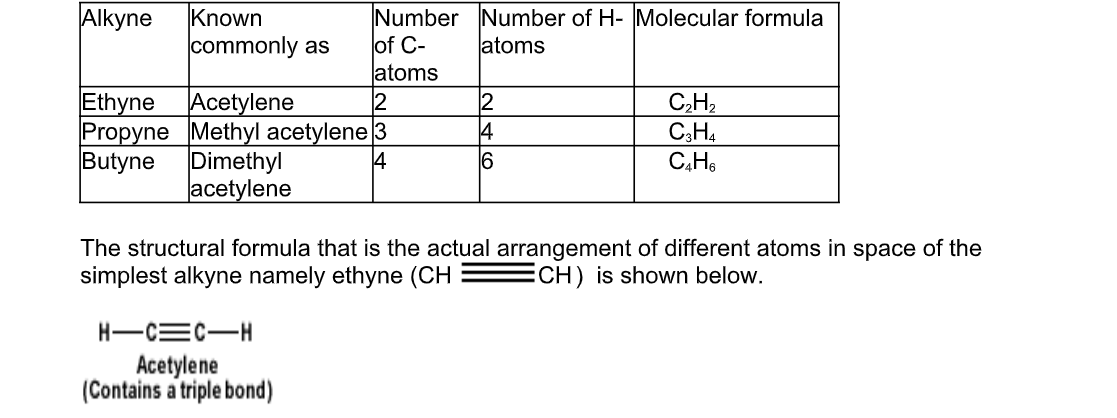
Hydrocarbons that have two carbon atoms in a triple bond are called alkynes
They are unsaturated bonds
Their general formula is CnH2n-2 and their names are derived from the alkanes by changing the ending “ane’ of the alkane by “yne”, for example, ethyne, propyne, butyne, etc
The simplest of alkynes has two carbon atoms in triple bond and is called ethyne
The table below gives names of the first three alkynes
Chemical properties of ethyne
1. Combustion: Ethyne burns in air with a sooty flame
It forms carbon dioxide and water and gives out heat
The sooty flame is due to higher amount of carbon in ethyne than in methane
All the carbon atoms cannot get oxidized while burning this makes the flame sooty
But if ethyne is burnt with a proper control, for example, if the gas is made to pass through a small nozzle, then it gets ample air mixture to burn completely
This type of complete combustion is used for acetylene lamps in industries
Acetylene lamps produce very luminous non-sooty flame
Ethyne combined well with oxygen can burn to give a flame whose temperature is 3000°C
This oxy-acetylene flame is used for welding metals, where very high temperatures are required
2. Reactivity: Alkynes are more reactive than the alkanes or alkenes due to the presence of unsaturated bonds
Such a reaction is called addition reaction
In an addition reaction, the alkynes will become an alkane
For example if ethyne is reacted with chlorine, it becomes 1,1,2,2 tetra-chloro-ethane
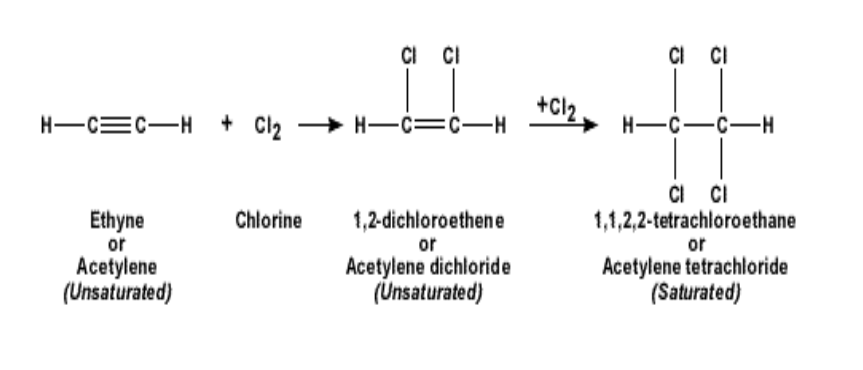
Similarly, addition reaction with bromine will give rise to 1,1,2,2, tetra-bromo-ethane
Bromine water decolorizes on reaction with ethyne
This is a prominent test for testing unsaturated nature of hydrocarbons
When hydrogen is added to ethyne, and heated in the presence of nickel, it becomes ethene and then proceeds to become ethane
The bonds become saturated
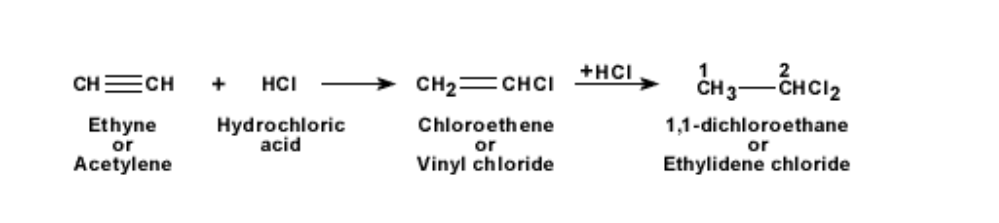
This is known as the process of hydrogenation
The addition of hydrogen to a double or triple bonded hydrocarbon leads to saturation of the bonds
When hydrochloric acid is added to ethyne, it becomes first chloro-ethene and then 1,1- dichloro-ethane
The reaction is shown below
Uses of ethyne
Hawkers use this as lamps
15.0.0 Nitrogen and Its Compounds Nitrogen
Nitrogen is a colourless and odourless gas, N2, which is insoluble in water
Although it does not support life, it is not poisonous
It reacts only with difficulty with other elements, requiring either high temperatures, a catalyst, or both, in order to form compounds
The most important of these are ammonia and ammonium salts, certain nitrogen oxides and nitric acid and its salts
Composition of air
The atmosphere is the gaseous envelope which surrounds the earth
This gas, air, is a mixture consisting of about 78% nitrogen and 21 % oxygen
Water vapour is present in variable amounts (up to 5%), and so the composition of unpolluted air is normally based on the dry gas mixture
The figures below are percentages of the normal constituents by volume
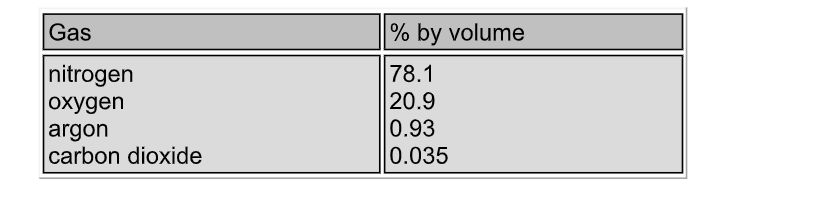
Nitrogen comprises about 78.1% of the earth’s atmosphere and it is the source of the commercial and industrial gas
Traces of other gases, notably He, Ne, Kr and Xe are also found, while near cities and industrial areas, all sorts of pollutants are also found
Air is liquefied, and the oxygen (about 20.9%) boiled off at -183 ºC, leaving liquid nitrogen (which boils at -196 ºC) behind
This process is known as fractional distillation
Preparation of Nitrogen from Air
Industrial preparation
The chief source of free nitrogen is atmospheric air and nitrogen is usually prepared from it
Air free from dust, water vapour and carbon dioxide is compressed in a compression chamber for liquefaction
Firstly, the pressure on the air is increased to about 200 atmospheres
It is then released through a spiral into a low-pressure area, where intense cooling of the air takes place
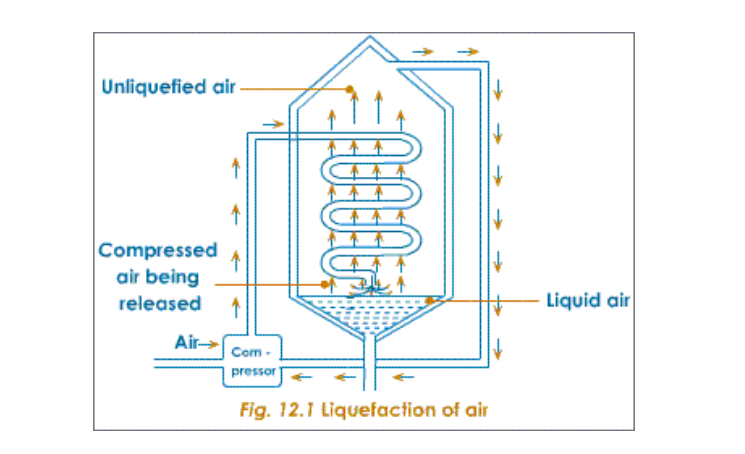
The cold air goes upwards and further cools the spiral that brings in a fresh batch of purified air
In this way the cold air in the spiral gets progressively cooled when released
This procedure continues and the cooling becomes gradually more and more intense
Ultimately, the cooling becomes so great that the temperature drops to nearly -200oC
At this temperature the air condenses to form liquid air (Nitrogen becomes liquid at -196oC)
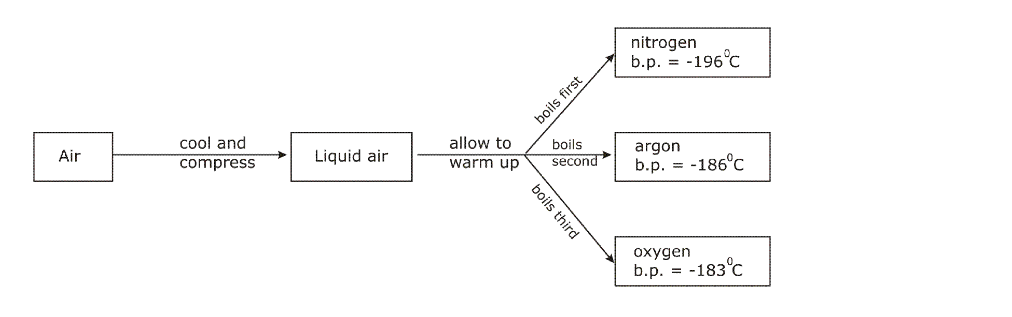
Liquid air is then led into a chamber, and allowed to warm up, by absorbing heat from the atmosphere
The boiling point of nitrogen is -196oC; when this temperature is reached, nitrogen starts boiling and the vapours (gas) is collected and packed
The liquid left behind is mainly oxygen, which has a higher boiling point of 183oC
Prepararion from Ammonia and Ammonium Compounds
(i) By treating excess ammonia with chlorine, ammonium chloride and nitrogen are formed
The products obtained are bubbled through water
The vapours of ammonium chloride dissolve in the water while nitrogen is collected separately
(ii) By passing ammonia over heated metallic oxides like copper oxide and lead oxide
(Fig.12.3)
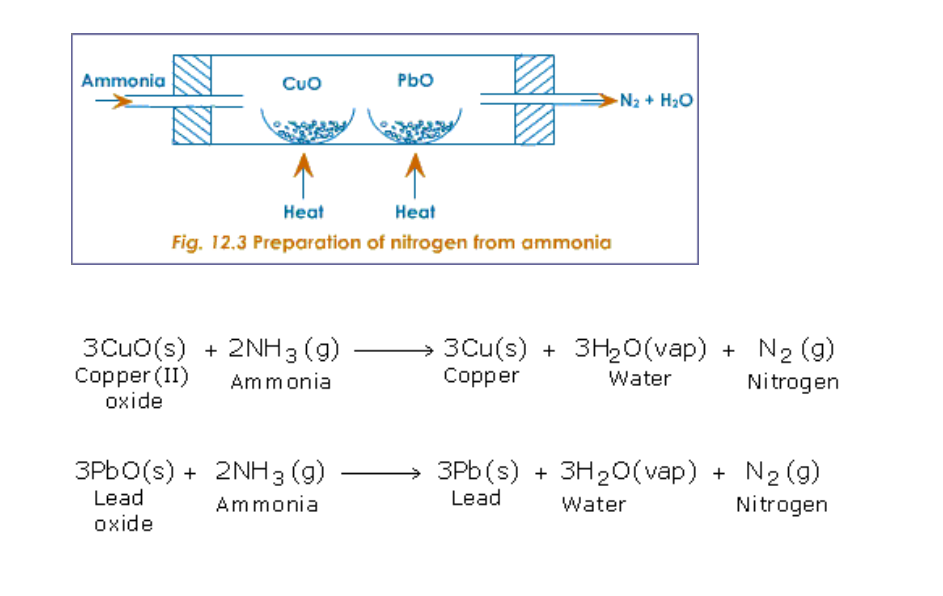
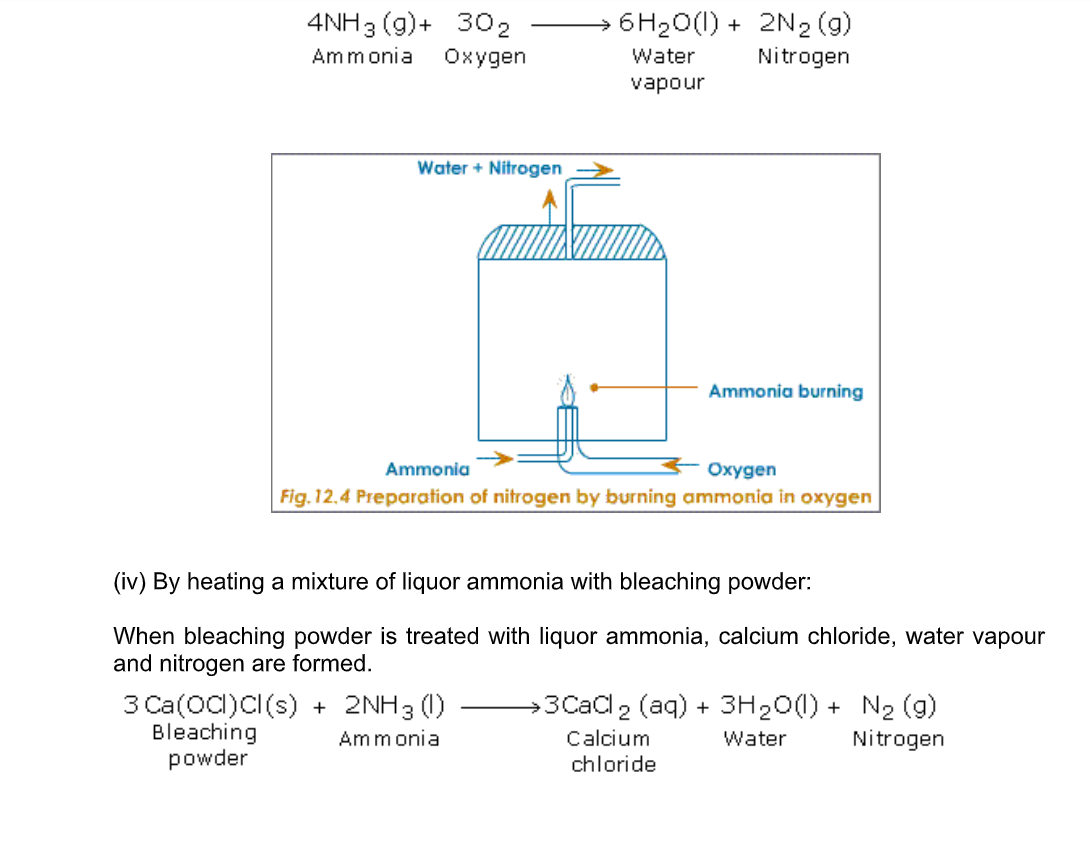
(iii) By burning ammonia in oxygen
Ammonia burns in oxygen to yield water vapour and nitrogen (Fig.12.4)
(v) By the action of heat on ammonium compounds: (ammonium dichromate)
Ammonium dichromate is an orange coloured crystalline substance
When heated it starts decomposing, with the evolution of heat
Sparks can be seen inside the test tube and therefore further heating is not necessary
The products of decomposition are, a green coloured solid of chromic oxide, water vapour and nitrogen gas (Fig.12.5)
However, collecting nitrogen by this method is difficult
As the reaction is accompanied by heat and light, it is quite violent
Also the green coloured fluffy chromic oxide gets sprayed all over and thrown out of the test tube
It is therefore difficult to control this reaction
Laboratory Preparation of Nitrogen
Method A
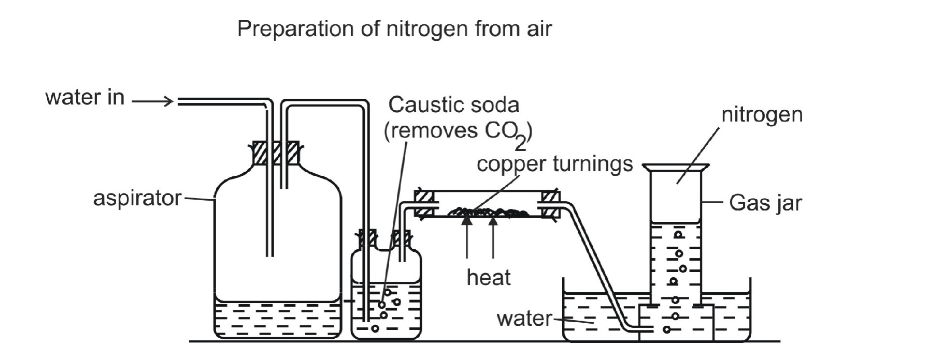
Nitrogen can be prepared from the air as shown
Air flows into the respirator and onto caustic soda which dissolves carbon dioxide gas
It is then passed through a heated combustion tube containing heated copper turnings which remove oxygen
Nitrogen is then collected over water
Traces of noble gases present in air still remain in the final product
Method B
Nitrogen can also be obtained by heating a mixture of sodium nitrite and ammonium chloride as shown
The gas collected by this method is purer than one in method A, even though it contains water vapour which could have been removed if the gas is passed through concentrated sulphuric acid before collection
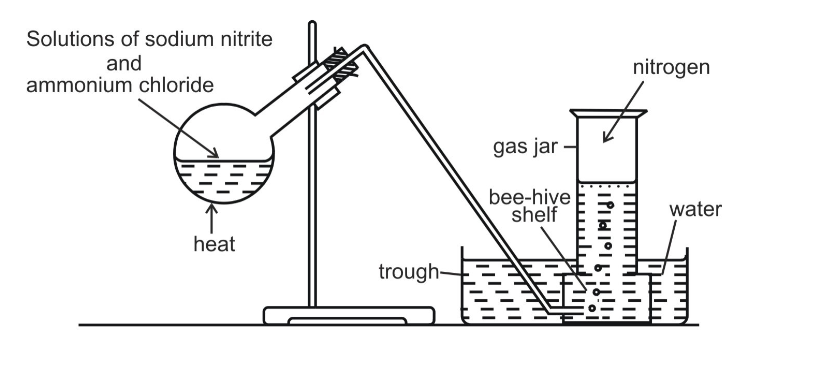
In the laboratory, nitrogen is prepared by heating a mixture of ammonium chloride and sodium nitrite and a small quantity of water
If ammonium nitrite is heated by itself it decomposes to produce nitrogen gas
However, this reaction is very fast and may prove to be explosive
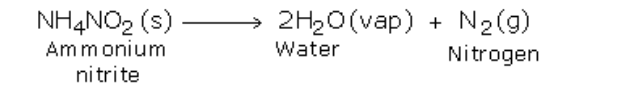
For safety, a mixture of ammonium chloride and sodium nitrite approximately in the ratio of 4:5 by mass, is heated mildly with a small quantity of water
The presence of water prevents ammonium chloride form subliming when heated
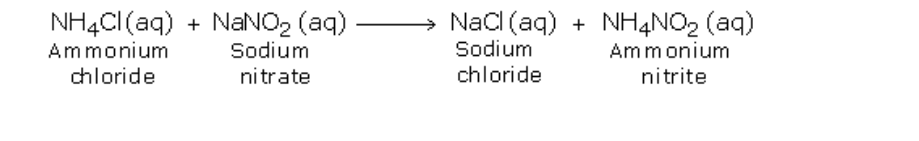
Initially, the two substances undergo double decomposition to form sodium chloride and ammonium nitrite
The ammonium nitrite so formed then decomposes to form nitrogen gas and water vapor
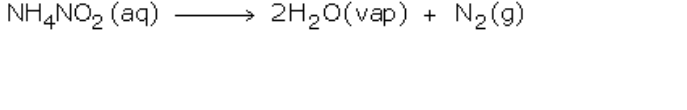
Nitrogen gas is collected by downward displacement of water
Uses of Nitrogen
(i) Nitrogen is used in high temperature thermometers where mercury cannot be used
This is because mercury boils at 356.7oC and hence cannot be used in such thermometers
A volume of nitrogen is enclosed in a vessel and introduced into the region of high temperature
Depending upon the temperature, expansion of the nitrogen volume takes place
Then applying the gas equation, the temperature is calculated
(ii) Nitrogen mixed with argon is used in electric bulbs to provide an inert atmosphere
It helps in prevention of oxidation and evaporation of the filament of the bulb, giving it a longer life
(iii) It is used to produce a blanketing atmosphere during processing of food stuff, to avoid oxidation of the food
It is also used when food is being canned, so that microorganisms do not grow
(iv) It is used in metal working operations to control furnace atmosphere and in metallurgy to prevent oxidation of red-hot metals
(v) Nitrogen in the air helps as a diluting agent and makes combustion and respiration less rapid
(vi) It is used by the chemical, petroleum, and paint industries to provide inactive atmosphere to prevent fires or explosions
(vii) It is used in the industrial preparation of ammonia, which is converted into ammonium salts, nitric acid, urea, calcium cyanamide fertilizers etc
(viii) Liquid nitrogen is used as a refrigerant for food, for storage of blood, cornea etc in hospitals
Meat, fish etc
, can be frozen in seconds by a blast of liquid nitrogen, which can provide temperatures below -196oC
(ix) Liquid nitrogen is used in scientific research especially in the field of superconductors
(x) Nitrogen is essential for synthesis of proteins in plants
Proteins are essential for synthesis of protoplasm, without which life would not exist
(xi) Liquid nitrogen is used in oil fields, to extinguish oil fires
Summary
Physical Properties
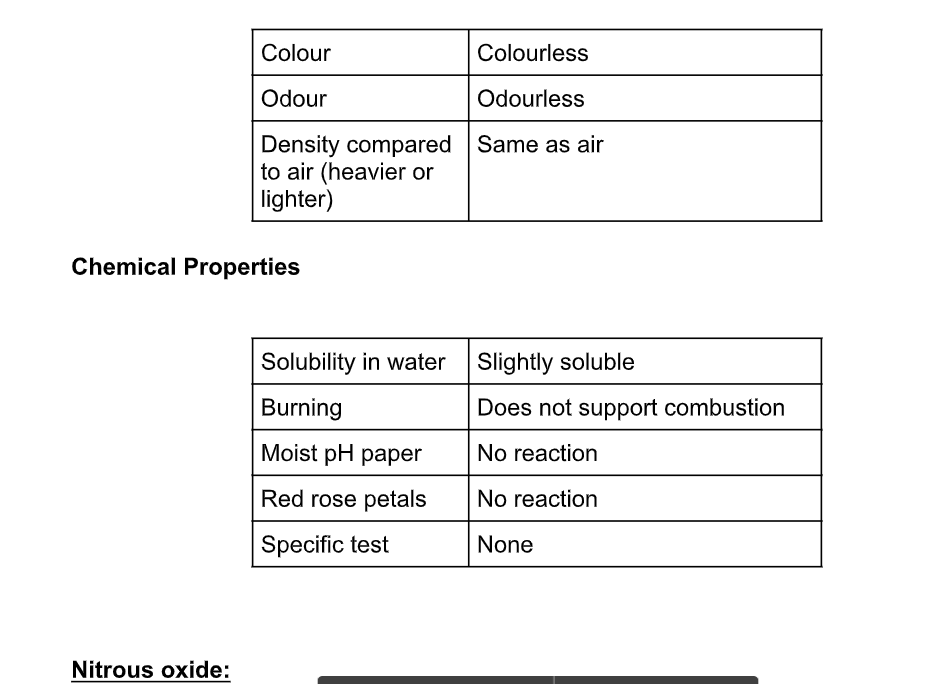
Nitrous oxide is a linear molecule
It has a boiling point of -88 ºC, and a melting point of -102 ºC
It is colourless and has a faintly sweet smell
It is used as an anaesthetic, popularly called laughing gas
Nitric oxide:
Nitric oxide, NO, may be prepared by the action of dilute nitric acid on copper:
3Cu + 8HNO,3 3Cu (NO3 )2 +2NO + 4H2 O It is a colourless gas, insoluble in water, which reacts with oxygen to form the brown gas nitrogen dioxide, NO3: O3 NO + O3 2NO3
Nitrogen dioxide:
Nitrogen dioxide, NO2 is a planar molecule
3
Cu (NO3)2+ 2H2O + 2NO2 or by the decomposition of heavy-metal nitrates, such as lead nitrate: 2Pb(NO3)2 2PbO + 4NO2 + O2 At temperatures below 100 ºC, it forms dinitrogen tetroxide, N2O4: 2NO2 N2O4 (REVERSIBLE ARROW)
Nitrogen dioxide will support combustion, as shown by the fact that a glowing splint of wood will ignite in this gas
Ammonia
Ammonia is a colorless gas
It has a characteristic pungent odor
It is bitter to taste
Its vapor density is 8.5 Hence it is lighter than air (vapor density of air = 14.4)
When cooled under pressure ammonia condenses to a colorless liquid, which boils at -33.4oC
When further cooled, it freezes to a white crystalline snow-like solid, which melts at -77.7oC
Ammonia is one of the most soluble gases in water At 0oC and 760 mm of Hg pressure one volume of water can dissolve nearly 1200 volumes of ammonia
This high solubility of ammonia can be demonstrated by the fountain experiment
Preparation of Ammonia
By Heating any Ammonium Salt with an Alkali
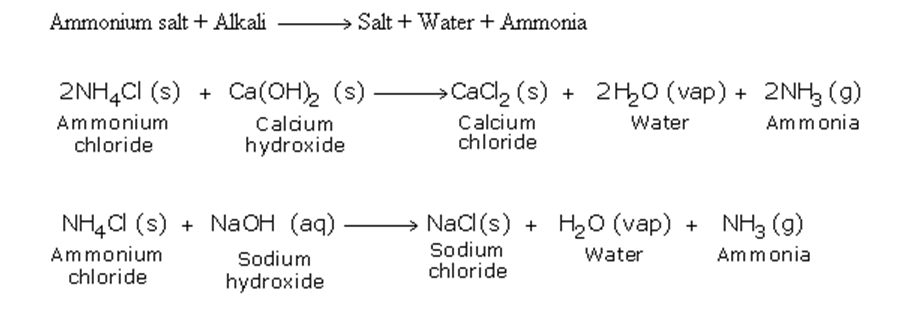
In the laboratory, ammonia is usually prepared by heating a mixture of ammonium chloride and slaked lime in the ratio of 2 : 3 by mass
The arrangement of the apparatus is shown in the figure 6.2 As ammonia is lighter than air, it is collected by the downward displacement of air
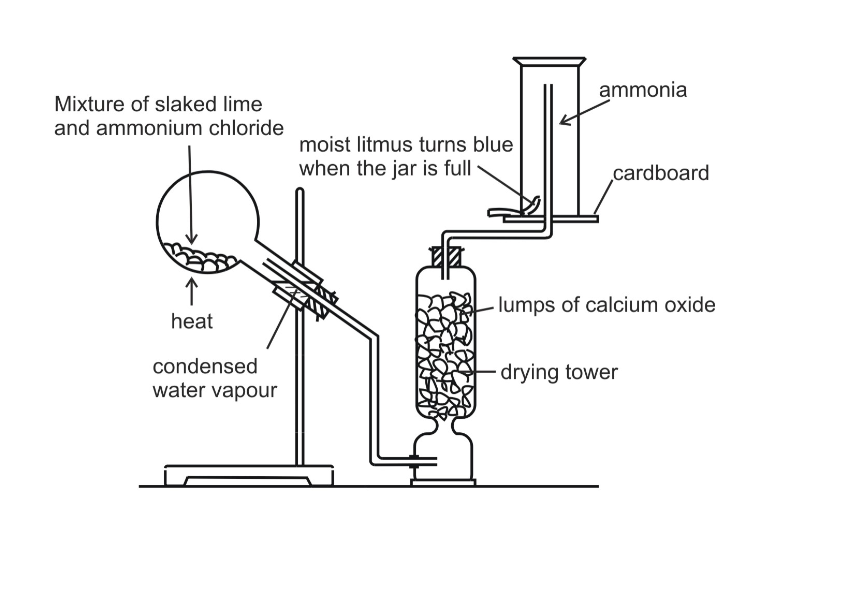
Drying of Ammonia
The drying agent used for ammonia is quick lime
Other drying agents such as concentrated sulphuric acid or phosphorus (V) oxide or fused calcium chloride cannot dry an alkaline gas like ammonia
Sulphuric acid and phosphorus (V) oxide are both acidic
They react with ammonia, forming their respective ammonium salt
Industrial Preparation of Ammonia
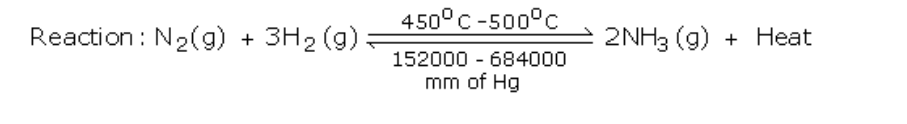
Haber’s Process
Ammonia is manufactured by Haber’s process using nitrogen and hydrogen (Fig.6.4)
Reactants: Nitrogen gas -1 volume and hydrogen gas -3 volumes
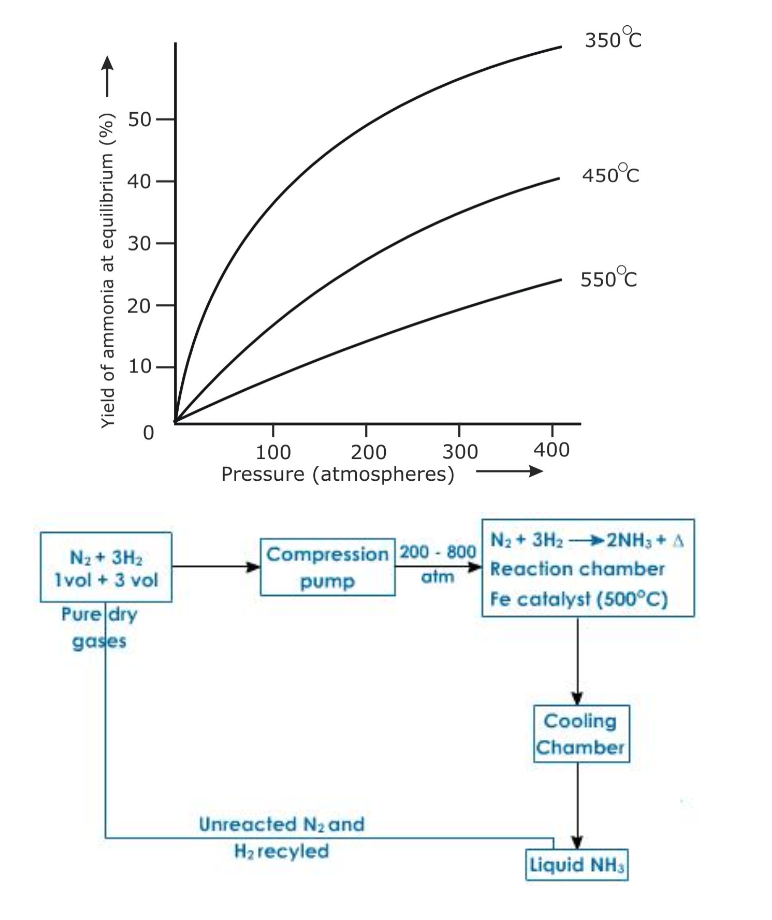
Conditions
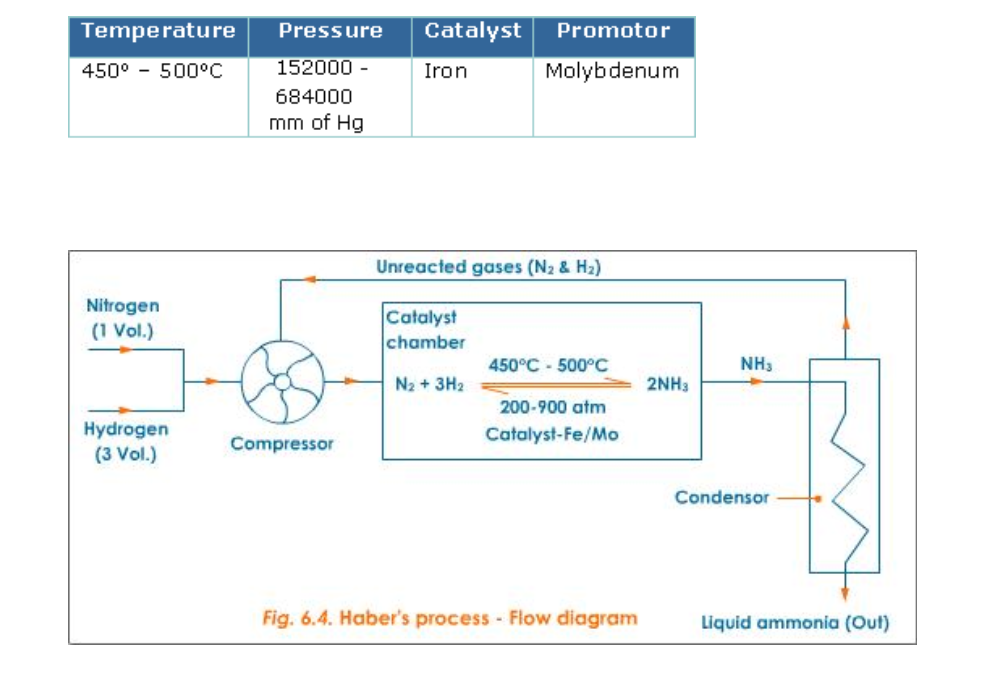
The reaction in Haber’s process is exothermic and so external heating is not required once the reaction starts
Lowering the temperature to 450o – 500oC favours the reaction, but lowering the temperature below 450o – 500oC brings down the yield
Nitrogen is obtained in large scale from air
Air free from dust and carbon dioxide is cooled under high pressure and low temperature to about 200oC and then allowed to warm
As nitrogen has lower boiling point (-169oC) as compared to oxygen (-183oC) it turns to gas leaving oxygen in liquid state
Nitrogen can also be obtained by heating a ammonium nitrite (in small amounts)
Chemical Properties of Ammonia
Combustibility
Ammonia is neither combustible in air nor does it support combustion
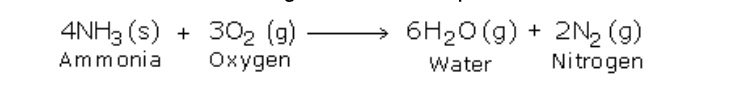
However it burns in oxygen with a greenish-yellowish flame producing water and nitrogen
a) Burning of Ammonia in Oxygen
Activity
Set the apparatus as shown in figure 6.7
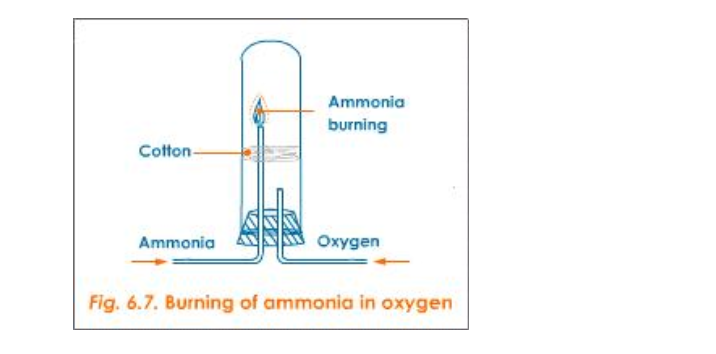
Firstly, when ammonia is passed through the longer tube and is made to ignite, it does not catch fire
Then oxygen is sent through the shorter tube
Now when ammonia is ignited, it catches fire and the following reaction takes place:
Although the products formed in the above reaction are insignificant, it is an extremely important reaction from viewpoint of industry
This is because in the presence of platinum, catalytic oxidation of ammonia can take place to give various important products
b) Catalytic Oxidation of Ammonia
The platinum coil is heated at 800oC in a combustion tube till it becomes white hot
Then ammonia and oxygen are passed through the tube
Under these conditions and in the presence of the catalyst, ammonia combines with free oxygen or oxygen of the air, to form nitric oxide and water vapour
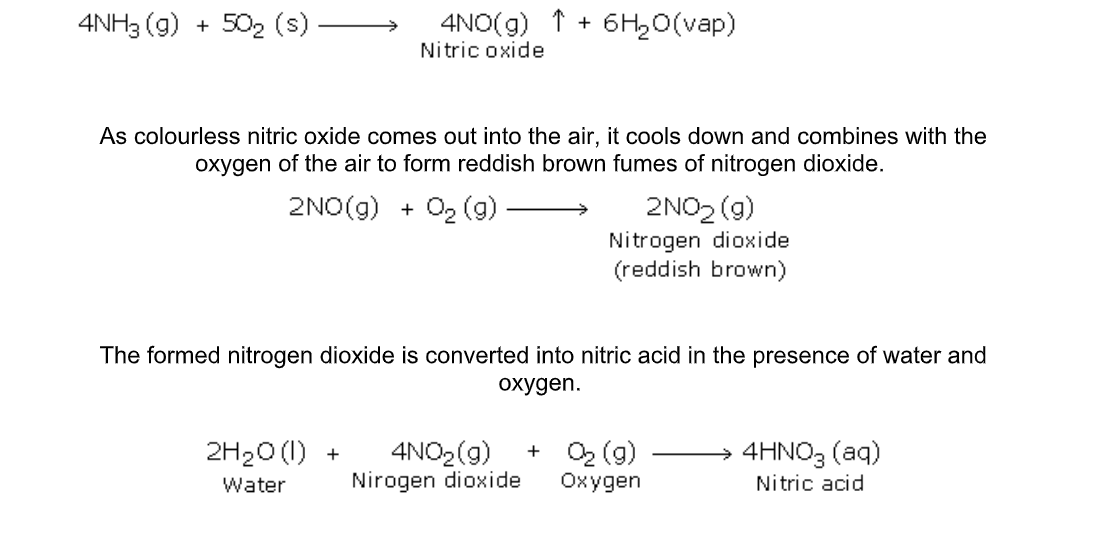
The importance of the above reactions lies in the production of nitric acid, which is a very important industrial product
Basic Nature
Absolutely dry ammonia or pure liquefied ammonia is neutral
In the presence of water however, it forms ammonium hydroxide, which yields hydroxyl ions
As a result of this reaction, it exhibits basic nature
It is a weak base and is perhaps the only gas that is alkaline in nature
Ammonia is an alkaline gas
When damp red litmus paper is introduced into the gas, it turns blue due to the presence of hydroxide ions as shown in the equation above
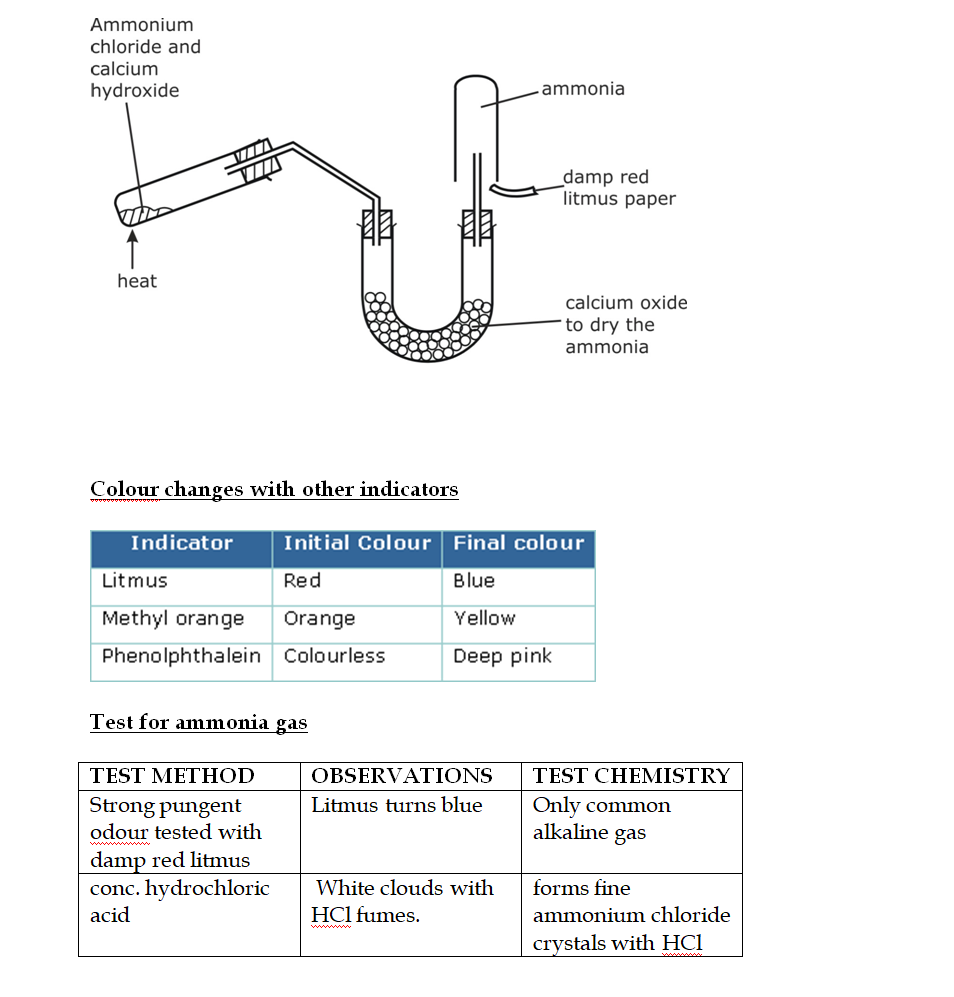
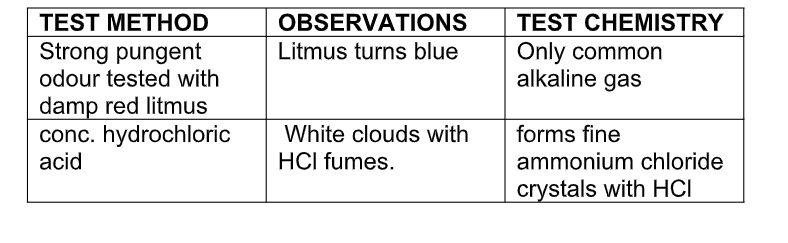
Test for ammonia gas
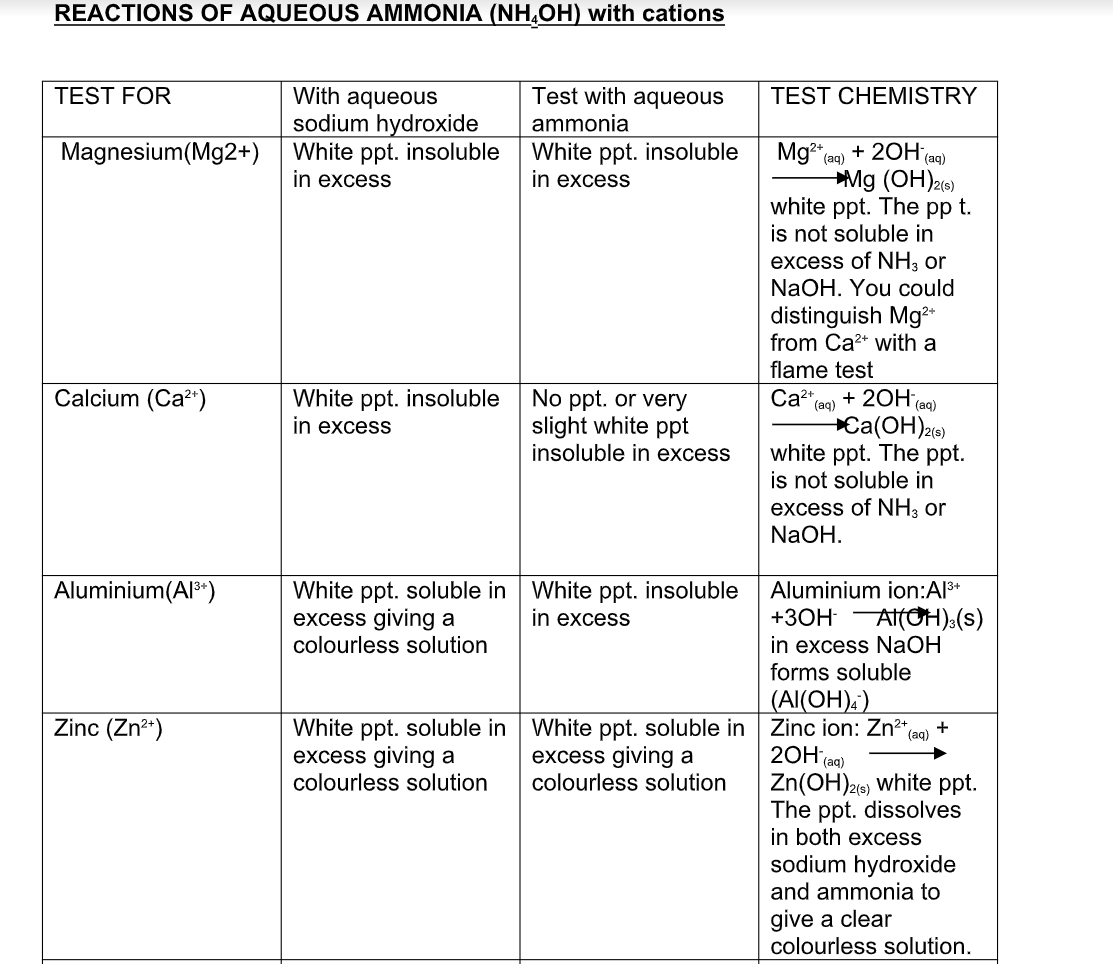
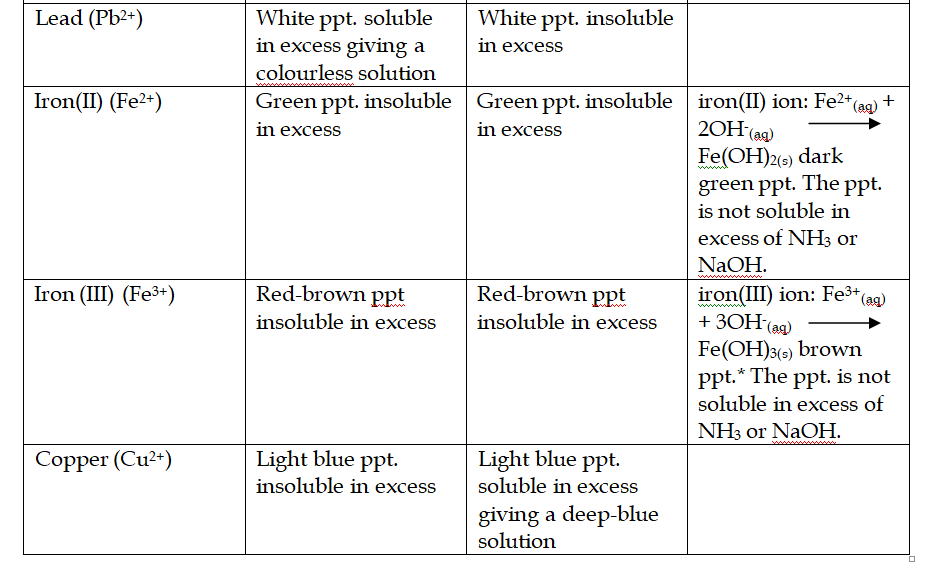
Reactions of Aqueous Ammonia (NH4OH) With Cations
Ammonia as a reducing agent
Heated dry ammonia gas can reduce copper (II) oxide to pure copper
This reaction can be used to prepare nitrogen
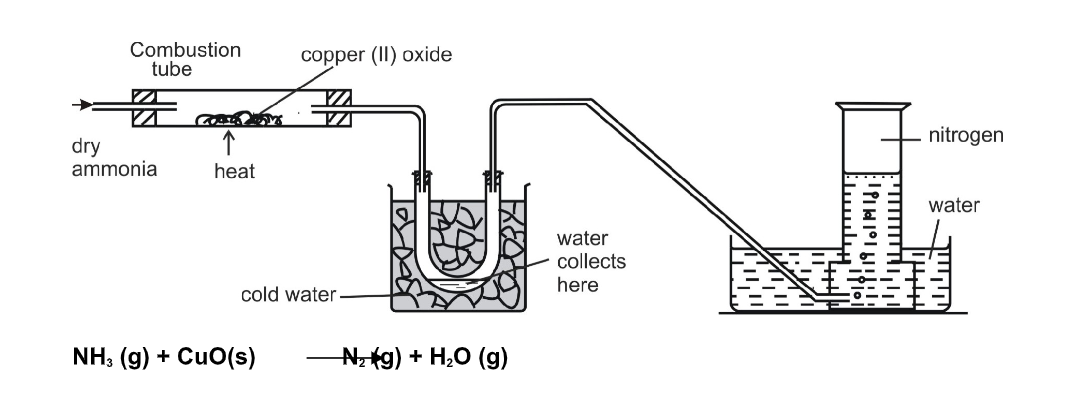
The gas passes through a U- tube surrounded by cold water which contains some melting ice
This helps to condense the vapour produced to liquid water
Nitrogen is finally collected by downward displacement of water
Fountain experiment
Fill a clean dry round-bottomed flask with dry ammonia, close it by a one holed stopper, through which a long jet tube is introduced
The free end of the tube is dipped into a trough of water as shown
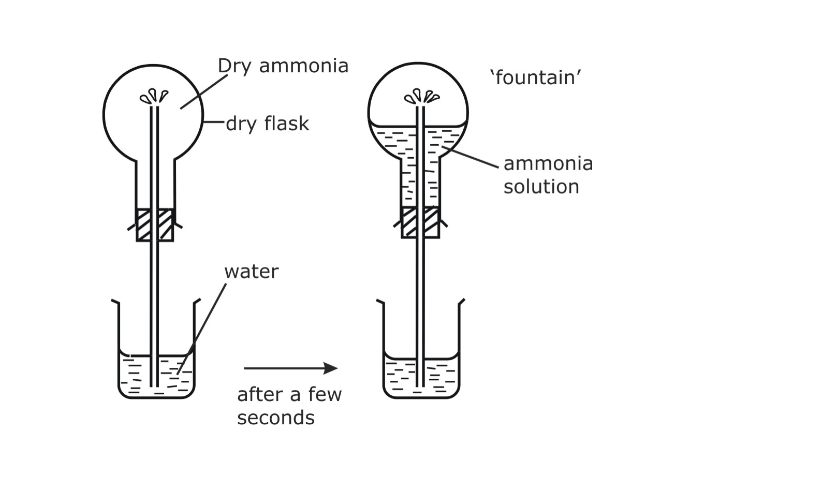
Add two or three drops of an acid and a small quantity of phenolphthalein to the water in the trough
This water is colorless
Pour a small quantity of spirit or ether on a layer of cotton and place it over the inverted flask
Due to the cooling effect produced by the process of evaporation of spirit or ether, the ammonia gas contracts a little and as a result, small quantity of the water gets sucked up
As soon as this water enters the flask, the ammonia dissolves in it, forming a partial vacuum
As a result of it, water rushes in and comes out of the tube as a jet of fountain
The color of the water turns deep pink
The properties of ammonia
Dissolving Ammonia in Water
Due to its high solubility, ammonia cannot be passed through water like many other gases
Ammonia is dissolved in water, as shown below
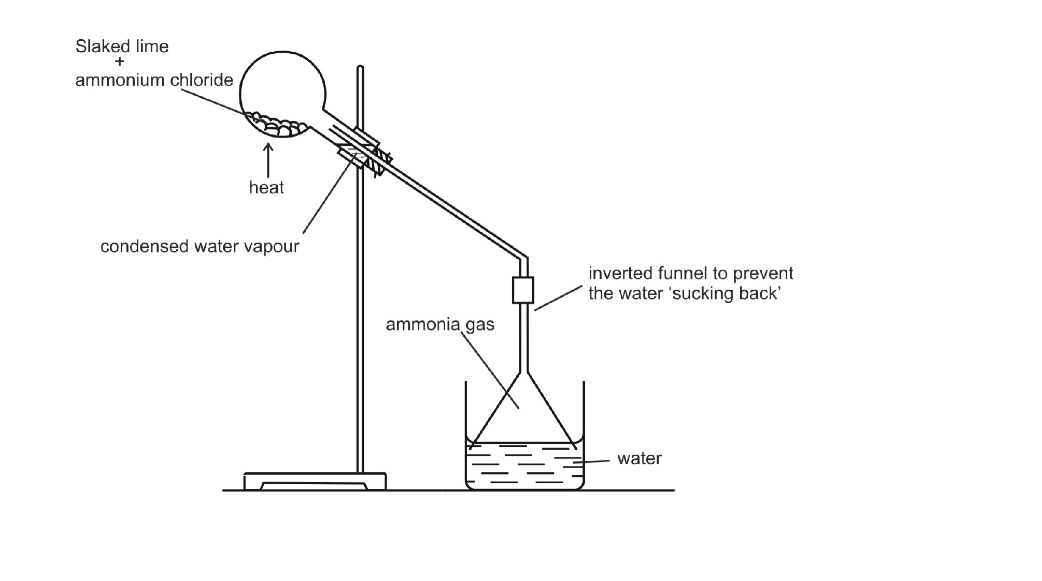
This arrangement is called funnel arrangement and its principle is the same as that discussed for HCl gas
The funnel arrangement prevents back suction of water, which can cause damage to the apparatus used
It provides larger surface area for dissolution of ammonia
A very strong solution of ammonia in water is called liquor ammonia
Ammonia can be obtained from it by boiling
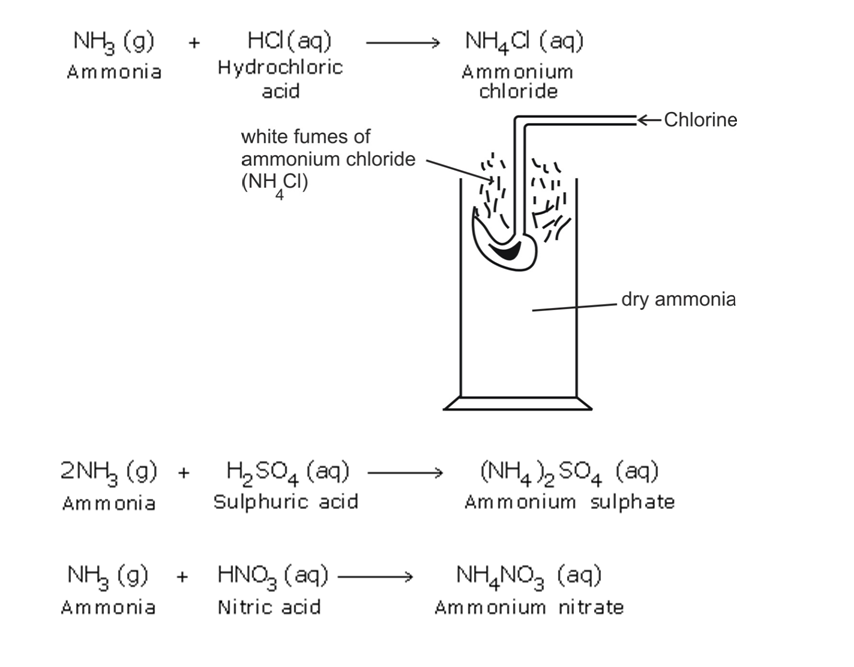
Action with Acids
Ammonia reacts with the acids to form their respective ammonium salts
The ammonium salts appear as white fumes
Ammonia gas + acid -> ammonium salt
Uses of Ammonia
Following are the chief uses of ammonia:
1) Ammonia is used in the industrial preparation of nitric acid by Ostwald’s process
2) Fertilisers, such as ammonium sulphate, ammonium nitrate, ammonium phosphate, urea etc
are manufactured with the help of ammonia
3) It is used in the manufacture of other ammonium salts, such as ammonium chloride, ammonium carbonate, ammonium nitrite etc
4) It finds use in the manufacture of nitrogen compounds such as sodium cynamide, plastics, rayon, nylon, dyes etc
5) It is used in the manufacture of sodium carbonate by Solvay’s process
(Ammonia and carbon dioxide are treated with aqueous sodium chloride, crystals of sodium hydrogen carbonate are formed
They are heated to yield sodium carbonate)
6) Ammonia acts as refrigerant in ice plants
Evaporation of a liquid needs heat energy
About 17g of liquid ammonia absorb 5700 calories of heat from the surrounding water
This cools the water and ultimately freezes it to ice
7) Ammonia is used to transport hydrogen
Hydrogen is dangerous to transport, as it is highly combustible
So it is converted to ammonia, liquefied, transported and then catalytically treated to obtain hydrogen
8) Many ammonium salts are used in medicines
Inhaling the fumes produced by rubbing ammonium carbonate in the hands can revive people who have fainted
9) It is used as a cleansing agent
Ammonia solution emulsifies fats, grease etc
so it can be used to clean oils, fats, body grease etc from clothes
It is also used to clean glassware, porcelain, floors etc
10) It is used as laboratory reagent
Nitric acid:
Nitric acid is produced industrially from ammonia by the Oswald process
It is a strong acid, converting bases to salts called nitrates: CuO + 2HNO3 Cu(NOO3)2 + HO2O
Copper(11) nitrate
NaOH + HNOO3 NaNOO3 + HO2O
Sodium nitrate
Nitric acid is also a strong oxidizing agent and may be reduced to nitric oxide or nitrogen dioxide:
Cu + 4HNOO3 Cu (NOO3)O2 + 2H2O + 2NO2 3Cu + 8HNOO3 3Cu (NOO3)O2 + 2NO +4HO2O Pure nitric acid slowly decomposes to form water, nitrogen dioxide and oxygen
This causes the nitric acid to become yellow
The process is accelerated on heating: 4HNOO3 2H2O + 4NOO2 + OO3
Oswald Process
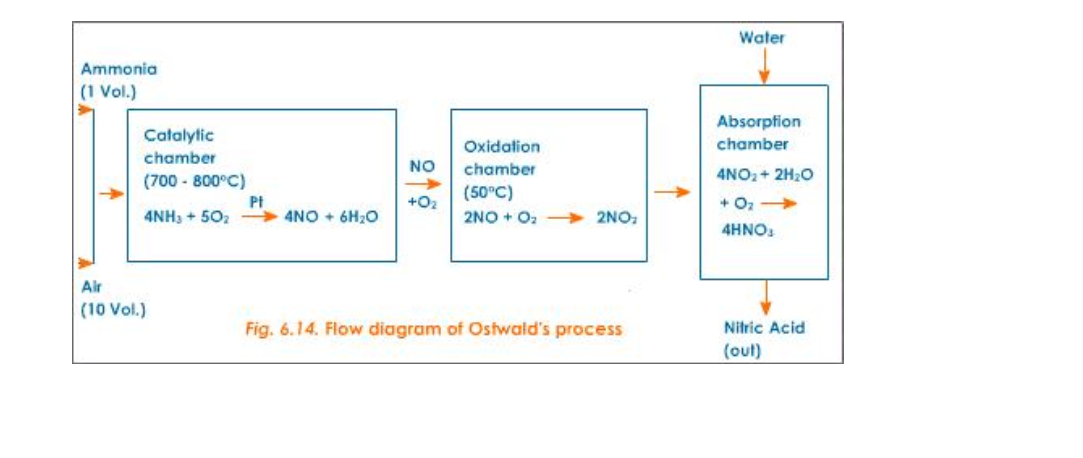
Nitric acid is prepared in large scale from ammonia and air (Fig.6.14)
Reactants
Pure dry ammonia (1 volume) and air (10 volumes)
Reactions
1) 1st step – Catalytic oxidation of ammonia to form nitric oxide
2) 2nd step – Oxidation of nitric oxide to nitrogen dioxide
3) 3rd step – Absorption of nitrogen dioxide in water to give nitric acid
Catalyst
Platinum (for oxidation of NH3)
Temperature
700o – 800oC
Reactions of Nitric Acid
Cuprous Oxide, Cu2O reacts with dilute Nitric Acid, HNO3, in the cold to form a solution of Cupric Nitrate, Cu (NO
)2, and Copper, Cu
Cu2O + 2 HNO3 Cu (NO3)2 + Cu + H2O Cuprous Oxide, Cu2O reacts with concentrated Nitric Acid, HNO3, or with dilute Nitric Acid, HNO3, on heating, when the Cuprous Oxide, Cu2O dissolves with evolution of Nitric Oxide, NO
3Cu2O + 14HNO3 6Cu (NO3)2 + 2NO + 7H2O Dinitrogen Pent oxide, N2O5, is best prepared by dehydrating concentrated Nitric Acid, HNO3, by Phosphorus Pent oxide, P2O5
2 HNO3 + P2O5 N2O5 + 2 HPO3 Nitric Oxide, NO is prepared by the action of Copper, Cu, or Mercury, Hg, on dilute Nitric Acid, HNO3, and was called Nitrous Air
3 Cu + 8 HNO3 3 Cu (NO3)2 + 2 NO + 4 H2O
Nitrogen dioxide, NO2, is a mixed acid anhydride and reacts with water to give a mixture of nitrous and nitric acids
2 NO2 + H2 HNO2 + HNO3
If the solution is heated the nitrous acid decomposes to give nitric acid and nitric oxide
3 HNO2 HNO3 + 2 NO + H2O
Sulphur Dioxide, SO2, and Nitrogen Oxides, NOx, are toxic acidic gases, which readily react with the Water, H2O in the atmosphere to form a mixture of Sulphuric Acid, H2SO4, Nitric Acid, HNO3, and Nitrous Acid, HNO2,
The dilute solutions of these acids which result give rain water a far greater acidity than normal, and is known as Acid Rain
Nitrates are the salts of nitric acid, and are strong oxidising agents
The Oswald Process is the tree stage process by which Nitric Acid, HNO3, is manufactured
Firstly, Ammonia, NH3, is oxidised, at high temperature (900 0C
) over a platinum-rhodium catalyst, to form Nitrogen Monoxide, NO
4 NH3 (g) + 5O2 (g) 4 NO (g) + 6H2O
The Nitrogen Monoxide, NO, cools and reacts with oxygen, O2, to produce Nitrogen Dioxide, NO2
2 NO (g) + O2 2 NO2 (g)
Finally, the Nitrogen Dioxide, NO2 reacts with Water and Oxygen, O2, oxygen to produce Nitric Acid,
4 NO2 (g) + 2 H2O (l) + O2 4 HNO3 (l)
Cu2O + 2 HNO3 Cu (NO3)2 + Cu + H2O Cuprous Oxide, Cu2O reacts with concentrated Nitric Acid, HNO3, or with dilute Nitric Acid, HNO3, on heating, when the Cuprous Oxide, Cu2O dissolves with evolution of Nitric Oxide, NO
3 Cu2O + 14 HNO3 6 Cu (NO3)2 + 2 NO + 7 H2O
Nitrates:
Salts of metals with nitric acid are called nitrates
Most nitrates are soluble in water
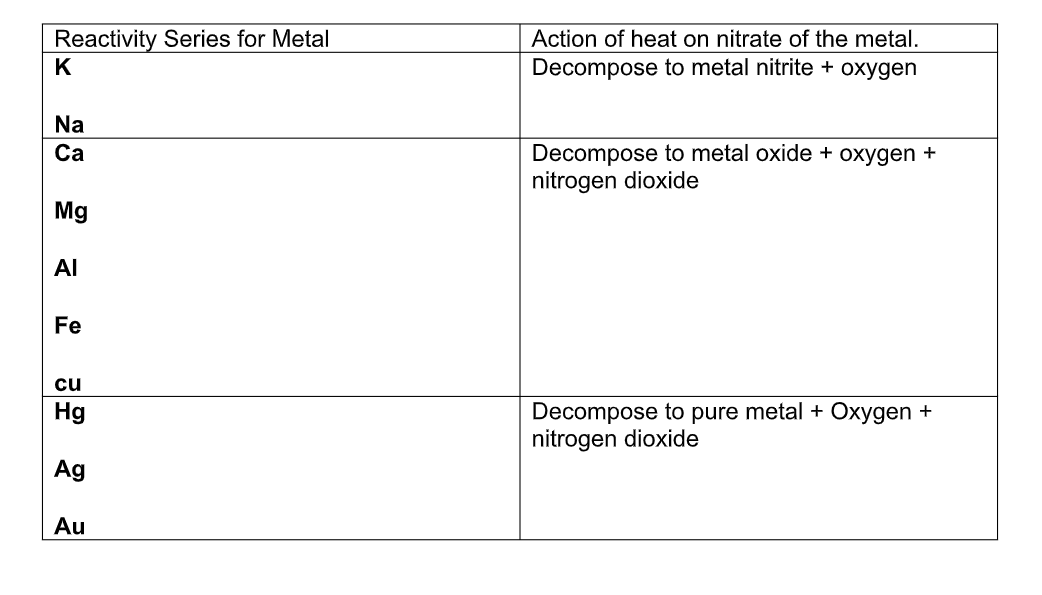
The nitrates of alkali metals form nitrites when strongly heated:
2NaNO3 2NaNO2 + O2
The nitrate of other metals decompose on heating to form nitrogen dioxide and the metal oxide, or, in the case of some metals such as silver and gold, the pure metal, nitrogen dioxide, and oxygen:
2Pb(NO3) 2PbO + 4NO2 + O2 2AgNO3 2Ag + 2NO2 + O2
Summary of Action of Heat on Nitrates
Generally compounds of very reactive metals such as sodium and potassium are more stable to heat than the metals lower down in the reactivity series of metals
Nitrogen pollution ??
16.0.0 Sulphur and Its Compounds
Sulphur
It takes the form of a yellow solid naturally and can be found in this state near volcanoes
Sulphur is also present in a number of metal ores, for example zinc blende (zinc sulphide, ZnS )
Sulphur has chemical symbol S
It has 16 protons and 16 neutrons
An atom of S is represented as 3216S
Sulphur is a non-metal and exists in the earth’s crust either as pure sulphur or as a metal-sulphide
Since S has 16 protons, it also has 16 electrons; the electronic configuration of S is 2, 8, 6
S is placed in Group VI A of the periodic table, just after phosphorus, and below oxygen
The reaction of S is similar to oxygen
Sulphur is found as a free element or in combined state in nature
Free sulphur is found in at a large depth below the earth’s surface
Metal sulphides such as Zn, Fe, Ag, Ca, Pb, Cu are found in abundant quantities
Mineral ores containing S are:
Sulphur is found as H2 S gas in petroleum gas, coal gas
H2S is the familiar pungent smell of onions
It is present in hair, eggs, many proteins and wool
Extraction of pure sulphur : Frasch process
Since sulphur in free state is found at depths of more than 150 to 300 meters below the earth’s surface, the method of extraction of sulphur differs from other metal or non-metal extractions
Sulphur’s relatively low melting point (115°C) is utilized in this process
This is known as the Frasch process
Here compressed super heated water (at 170°C) is pressed into a pipe which reaches up to the sulphur deposits
The sulphur here melts
Introducing hot compressed air through another pipe brings it up
The molten sulphur and water mixture is forced up and is collected in a settling tank
The sulphur is cooled and water is evaporated
The sulphur extracted in this way is more than 99% pure
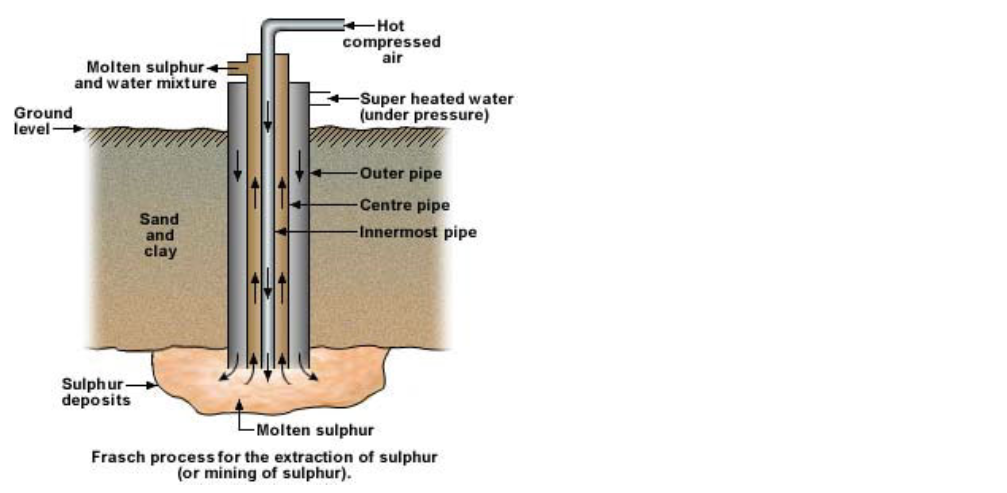
The sulphur obtained by Frasch process is a yellow and brittle solid or powder
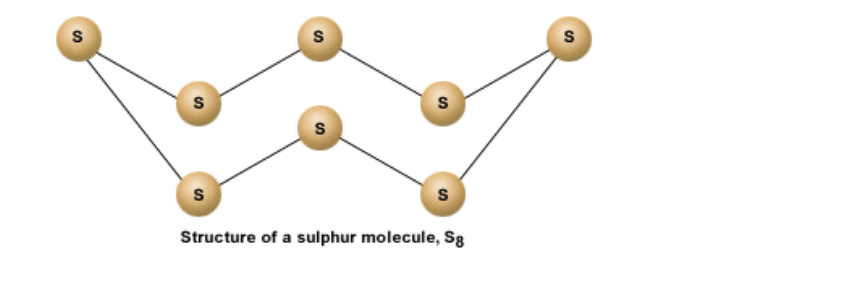
Physical properties of sulphur : Since S has 6 electrons in its outermost shell, it needs 2 more electrons to complete its shell
But S combines with 7 other atoms to make a sulphur molecule that has a total of 8 sulphur atoms
Thus each S atom shares 2 electrons with its neighboring atom
The bonds are covalent in nature
A molecule of sulphur is represented as S8
It is a ringed molecule
The structure is shown below
These large molecules each have many electrons , so the Van der Waals forces are quite strong and the melting point is quite high (119oC)
There are two ways of packing the sulphur rings, so solid sulphur exists in two crystalline forms, called rhombic and monoclinic
Allotropes of sulphur
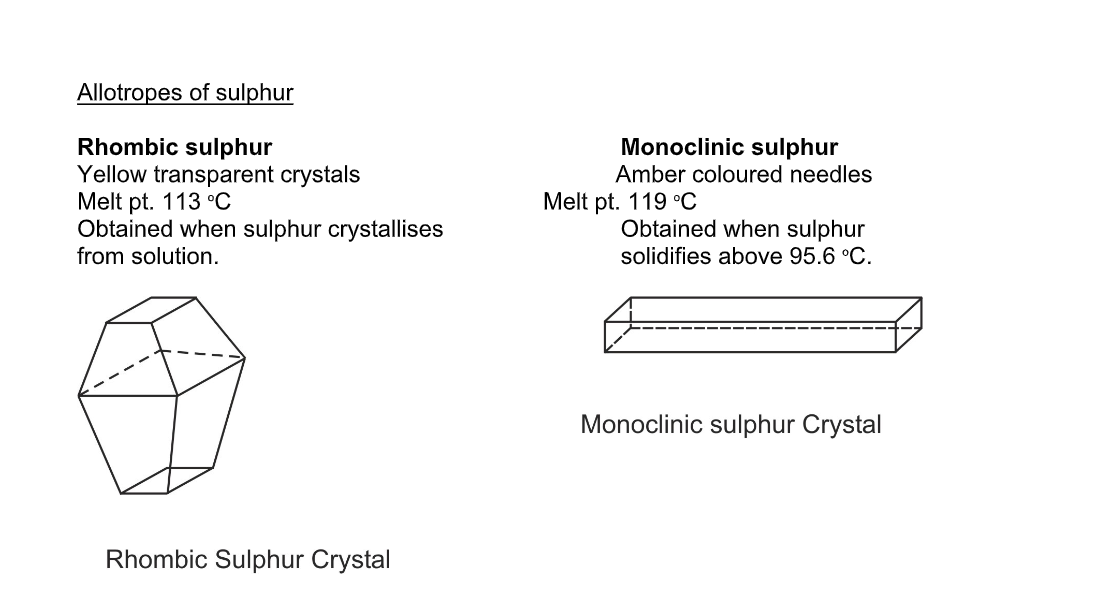
Sulphur is a yellow crystalline solid
It is tasteless and odourless
The melting point of S is 115°C
Sulphur is an insulator and is a poor conductor of heat and electricity
S is insoluble in water but is soluble in CS2
Sulphur forms covalent bonds and shows allotropic forms
The allotropes have different crystalline shapes such as rhombic and monoclinic
There is another allotrope which has no shape and is called plastic sulphur
Vapours of sulphur are pungent and although not poisonous, they can cause health problems to humans
Chemical properties of sulphur :
1. Valence : Since S has 6 electrons in its outer shell
Hence S does not give off its electrons easily
It readily forms covalent bonds to complete its outer shell
It shows variable valence of 2 or 6 S is quite a reactive element and forms oxides, chlorides and sulphides readily
2. Action of oxygen: Sulphur reacts with oxygen and burns with a blue flame
It forms sulphur dioxide which is a colourless gas having a pungent smell
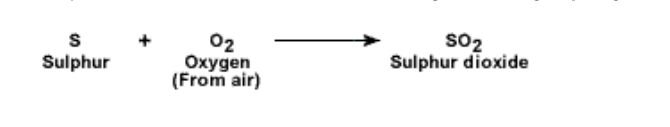
Sulphur dioxide forms an acidic solution, sulphurous acid, when dissolved in water i.e it turns damp blue litmus paper red
It will also react with oxidising agents to produce sulphate ions e.g Orange acidified dichromate (VI) ions are turned green and purple acidified manganese (VII) ions are turned colourless
3. S reacts with other non-metals also
In all cases sulphur has to be heated or boiled for the reaction to take place
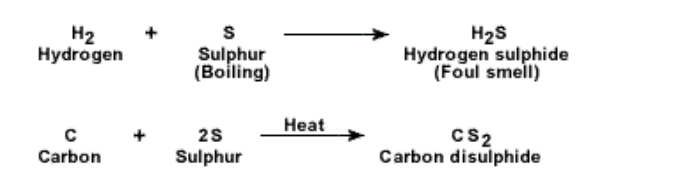
4. Reaction with metals : Heated S reacts with metals like Fe, Cu, Zn, Sb directly to give metal-sulphide
A mixture of powdered zinc and sulphur, when heated up to a high temperature, will react together to produce an extremely exothermic change
A few reactions are shown below

5. Reaction with acids : S is oxidized by strong concentrated oxidizing acids such as sulphuric acid and nitric acid
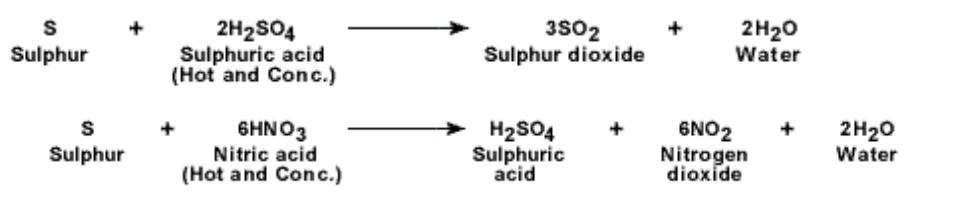
In both the reactions S acts as a reducing agent
Effect of heat on sulphur:
A sulphur molecule consists of 8 atoms in a ring form
When heated, S melts at 115°C and a pale yellow liquid is formed
The S8 ringed molecules are connected to other molecules in a long chain
On heating, the long chain breaks up
The individual molecules can slip over each other when melted
On further heating, the liquid becomes dark brown and viscous
When the temperature rises beyond 160°C, the intra-molecular bonds break
Sulphur boils at 444°C
At this temperature the large molecule breaks up into pieces of S2 molecule
This molecule is pale yellowish-brown in colour
The vapours of S contains S2 molecules
Vulcanization of rubber :
Natural rubber is a soft and sticky solid
Rubber is a long chain polymer made out of isoprene (2 – methyl butadiene) monomer
The long molecule forms a coil like structure
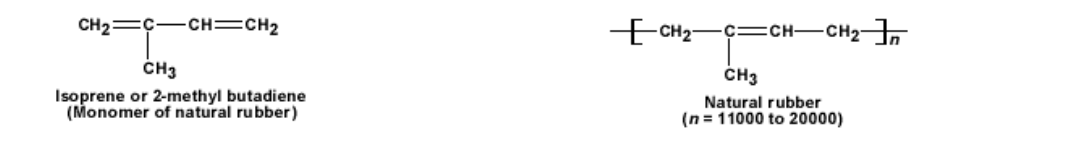
The unique property of rubber is that it is elastic
When rubber is stretched, the molecular bonds can be extended out
When released, the molecules coil back to their original shape
Natural rubber looses its rubber-like properties at temperatures above 60°C
Also its wear resistance and tensile strengths are low
The process of vulcanization can improve the quality of rubber
Raw rubber is heated with sulphur during vulcanization
This makes the rubber hard, more elastic and strong
During the process of vulcanization, the sulphur atoms attach themselves to extra loose bonds in the rubber molecule and also cross-link the molecules
The cross-linking locks the molecules in place and prevents slipping
Thus making the vulcanized rubber more strong
Vulcanized rubber is non-sticky and has higher elasticity
It does not loose its properties easily and can be used in a temperature range of – 40°C to 100°C
Uses of sulphur :
The Properties Of Sulphur:
Sulphur Dioxide
Moist sulphur dioxide (or sulphurous acid) is a reducing agent
This fact is used as a test for the detection of sulphur dioxide
1. There is a colour change from purple (pink in dilute solution) to colourless on the addition of the gas to a solution of potassium manganate (VII) (permanganate) 2MnO4- + 5SO
There is a colour change from orange to blue on adding the gas to a solution of potassium dichromate (VI)
Cr2O72- + 3SO2 +2H+ 2Cr3+ + 3SO42- + H2O Sulphurous acid and Sulphites Sulphur dioxide dissolves in water forming sulphuric (IV) acid (sulphurous acid)
SO2 (aq) + H2O (l) H2SO3 (aq) This is a weak dibasic acid and ionises producing hydrogen ions and sulphite SO32- ions
H2SO3 2H+ + SO32-
1. Sulphites give sulphur dioxide on heating with dilute acids
Na2SO3+ 2HCl NaCl + SO2 + H2O
2. With barium chloride they give a white precipitate of barium sulphite which is soluble in dilute hydrochloric acid
Ba2+ (aq) + SO32- (aq) BaSO3 (s) (White precipitate) BaSO3 (s) + 2HCl BaCl2 + SO2 (g) + H2O This reaction is used as a test for sulphite ions in solution
Sulphur Dioxide
Sulphur dioxide is a colourless gas, about 2.5 times as heavy as air, with a suffocating smell, faint sweetish odour
Occurrence Sulphur dioxide occurs in volcanic gases and thus traces of sulphur dioxide are present in the atmosphere
Other sources of sulphur dioxide are the combustion of the iron pyrites which are contained in coal
Sulphur dioxide also results from various metallurgical and chemical processes
Preparation of Sulphur Dioxide
Sulphur dioxide is prepared by burning sulphur in oxygen or air
S + O2 SO2
Sulphur dioxide is usually made in the laboratory by heating concentrated sulphuric acid with copper turnings
Cu + 2 H2SO4 CuSO4 + SO2 + 2 H2O
Sulphur dioxide is released by the action of acids on sulphites or acid sulphites (e.g by dropping concentrated sulphuric acid into a concentrated solution of sodium hydrogen sulphite)
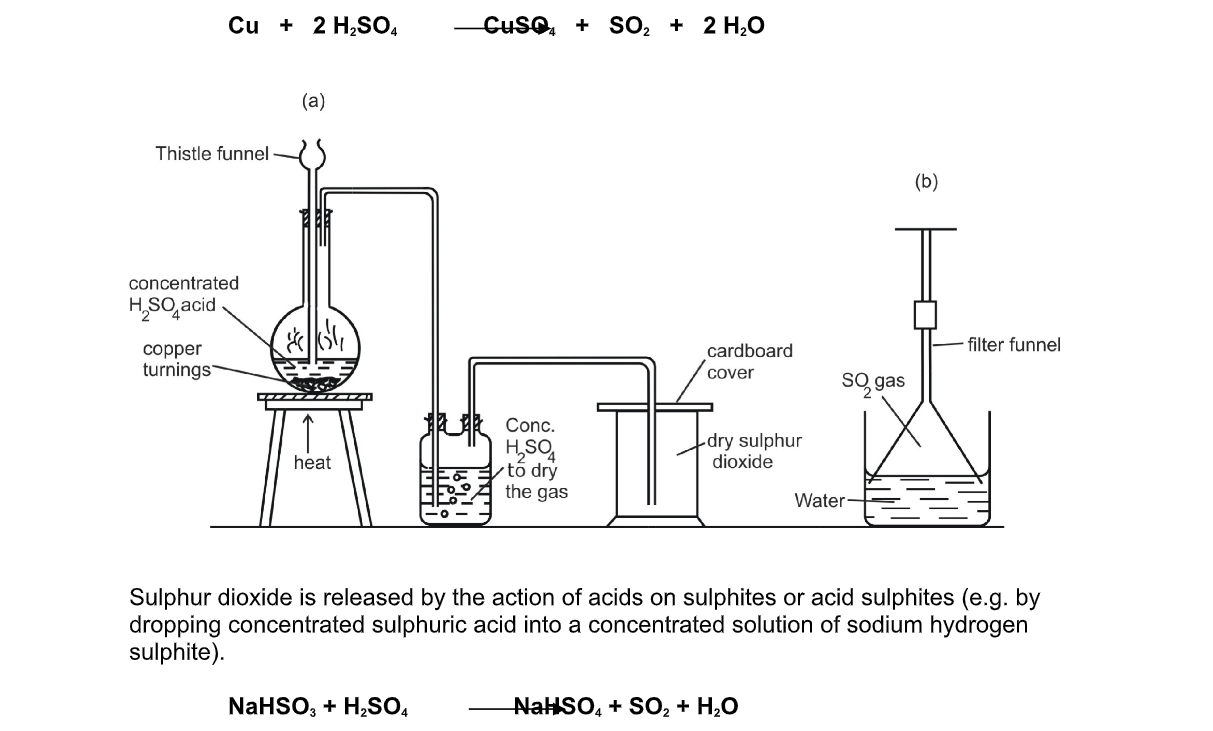
Sulphur dioxide is a colourless liquid or pungent gas, which is the product of the combustion of sulphur on air
Its melting point is -72.7 0C, its boiling point is -100C and its relative density is 1.43
Sulphur Dioxide is an acidic oxide which reacts with water to give sulphurous acid
SO2 + H2O H2SO3 Sulphur dioxide is a good reducing and oxidising agent
Summary
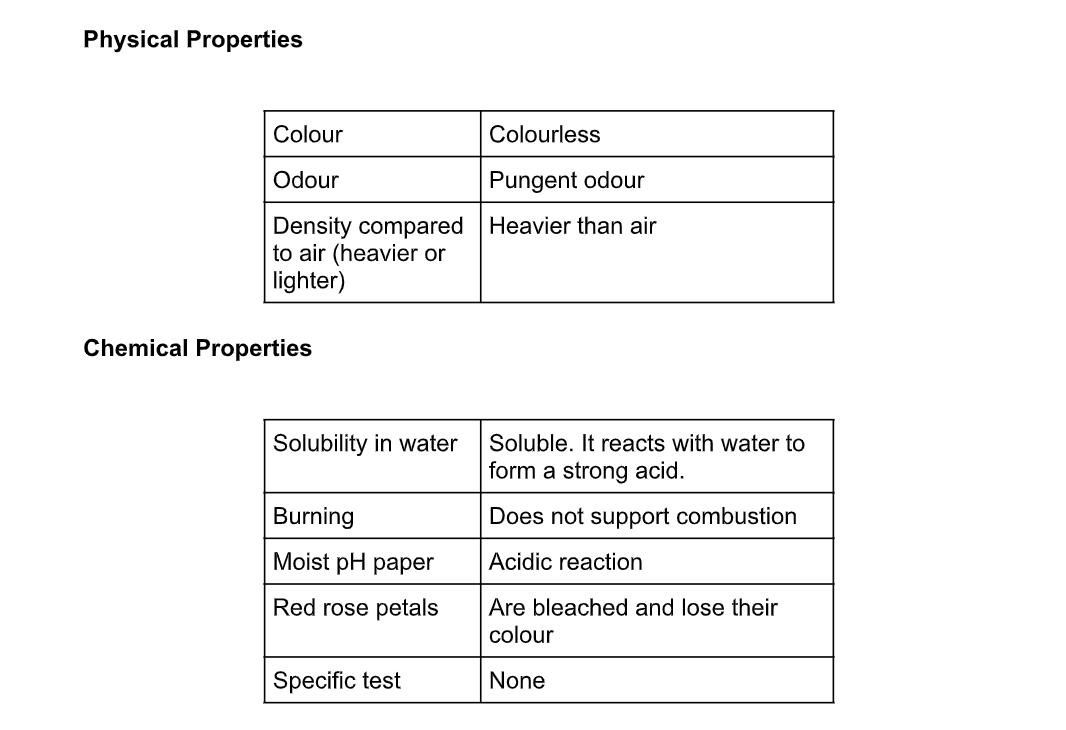
Uses of Sulphur Dioxide
a). Sulphur dioxide is a reducing agent and is used for bleaching and as a fumigant and food preservative
b). Large quantities of sulphur dioxide are used in the contact process for the manufacture of sulphuric acid
c). Sulphur dioxide is used in bleaching wool or straw, and as a disinfectant
d). Liquid sulphur dioxide has been used in purifying petroleum products
e). It is used as a bleaching agent
The Contact Process
This page describes the Contact Process for the manufacture of sulphuric acid, and then goes on to explain the reasons for the conditions used in the process
It looks at the effect of proportions, temperature, pressure and catalyst on the composition of the equilibrium mixture, the rate of the reaction and the economics of the process
A brief summary of the Contact Process
The Contact Process:
Making the sulphur dioxide
This can either be made by burning sulphur in an excess of air:
or by heating sulphide ores like pyrite in an excess of air:
In either case, an excess of air is used so that the sulphur dioxide produced is already mixed with oxygen for the next stage
Converting the sulphur dioxide into sulphur trioxide
This is a reversible reaction, and the formation of the sulphur trioxide is exothermic
A flow scheme for this part of the process looks like this:
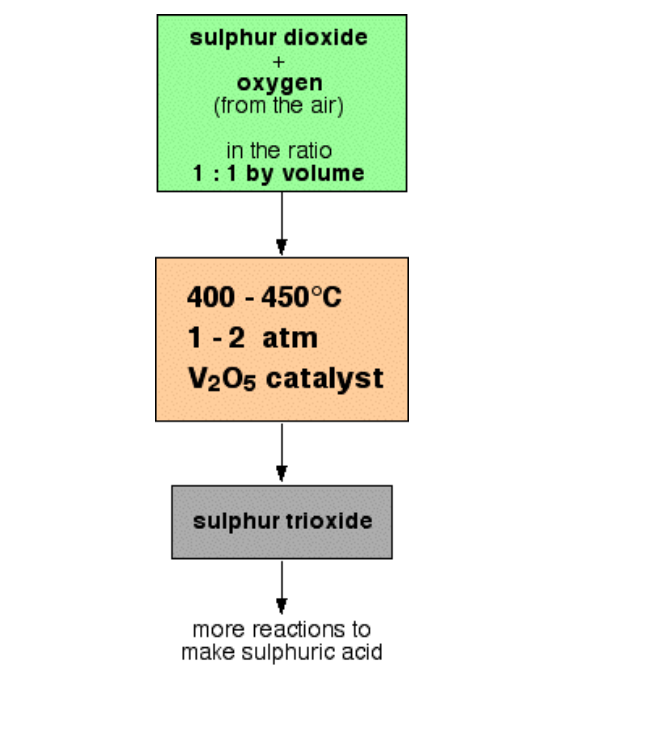
Converting the sulphur trioxide into sulphuric acid This can’t be done by simply adding water to the sulphur trioxide – the reaction is so uncontrollable that it creates a fog of sulphuric acid
Instead, the sulphur trioxide is first dissolved in concentrated sulphuric acid:
The product is known as fuming sulphuric acid or oleum
This can then be reacted safely with water to produce concentrated sulphuric acid – twice as much as you originally used to make the fuming sulphuric acid
Summary
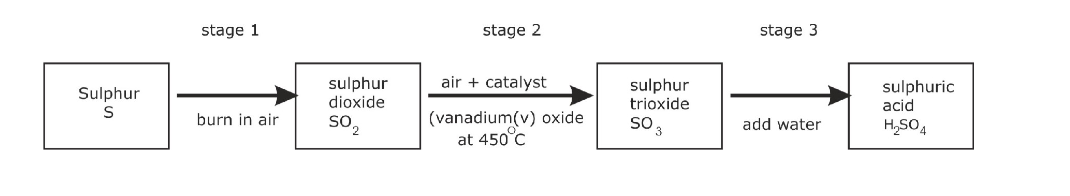
Explaining the conditions
The proportions of sulphur dioxide and oxygen
The mixture of sulphur dioxide and oxygen going into the reactor is in equal proportions by volume
Avogadro’s Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules
That means that the gases are going into the reactor in the ratio of 1 molecule of sulphur dioxide to 1 of oxygen
That is an excess of oxygen relative to the proportions demanded by the equation
According to Le Chatelier’s Principle, Increasing the concentration of oxygen in the mixture causes the position of equilibrium to shift towards the right
Since the oxygen comes from the air, this is a very cheap way of increasing the conversion of sulphur dioxide into sulphur trioxide
Why not use an even higher proportion of oxygen? This is easy to see if you take an extreme case
Suppose you have a million molecules of oxygen to every molecule of sulphur dioxide
The equilibrium is going to be tipped very strongly towards sulphur trioxide – virtually every molecule of sulphur dioxide will be converted into sulphur trioxide
Great! But you aren’t going to produce much sulphur trioxide every day
The vast majority of what you are passing over the catalyst is oxygen which has nothing to react with
By increasing the proportion of oxygen you can increase the percentage of the sulphur dioxide converted, but at the same time decrease the total amount of sulphur trioxide made each day
The 1: 1 mixture turns out to give you the best possible overall yield of sulphur trioxide
The temperature
Equilibrium considerations
You need to shift the position of the equilibrium as far as possible to the right in order to produce the maximum possible amount of sulphur trioxide in the equilibrium mixture
The forward reaction (the production of sulphur trioxide) is exothermic
According to Le Chatelier’s Principle, this will be favoured if you lower the temperature
The system will respond by moving the position of equilibrium to counteract this – in other words by producing more heat
In order to get as much sulphur trioxide as possible in the equilibrium mixture, you need as low a temperature as possible
However, 400 – 450°C isn’t a low temperature!
Rate considerations
The lower the temperature you use, the slower the reaction becomes
A manufacturer is trying to produce as much sulphur trioxide as possible per day
It makes no sense to try to achieve an equilibrium mixture which contains a very high proportion of sulphur trioxide if it takes several years for the reaction to reach that equilibrium
You need the gases to reach equilibrium within the very short time that they will be in contact with the catalyst in the reactor
The compromise
400 – 450°C is a compromise temperature producing a fairly high proportion of sulphur trioxide in the equilibrium mixture, but in a very short time
The pressure
Equilibrium considerations
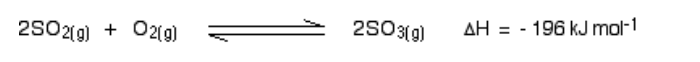
Notice that there are 3 molecules on the left-hand side of the equation, but only 2 on the right
According to Le Chatelier’s Principle, if you increase the pressure the system will respond by favouring the reaction which produces fewer molecules
That will cause the pressure to fall again
In order to get as much sulphur trioxide as possible in the equilibrium mixture, you need as high a pressure as possible
High pressures also increase the rate of the reaction
However, the reaction is done at pressures close to atmospheric pressure!
Economic considerations
Even at these relatively low pressures, there is a 99
5% conversion of sulphur dioxide into sulphur trioxide
The very small improvement that you could achieve by increasing the pressure isn’t worth the expense of producing those high pressures
The catalyst
Equilibrium considerations
The catalyst has no effect whatsoever on the position of the equilibrium
Adding a catalyst doesn’t produce any greater percentage of sulphur trioxide in the equilibrium mixture
Its only function is to speed up the reaction
Rate considerations
In the absence of a catalyst the reaction is so slow that virtually no reaction happens in any sensible time
The catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reactor
Properties of Sulphuric Acid
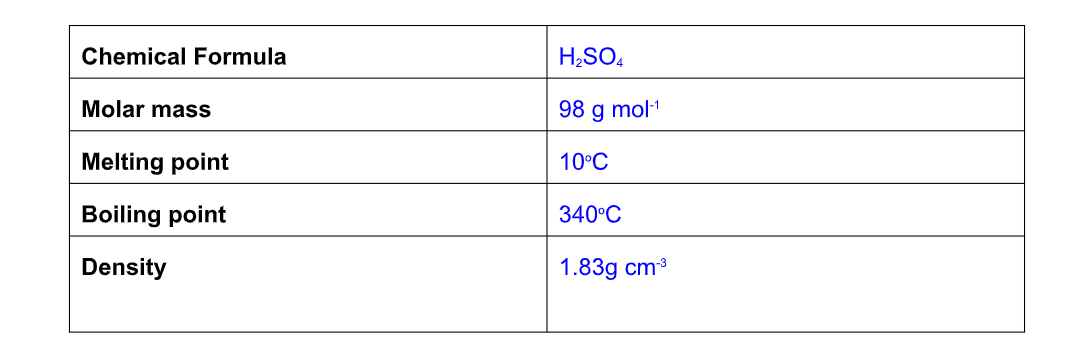
Sulphuric acid is a dense, oily liquid once known as oil of vitriol
Pure sulphuric acid is almost twice as dense as water (1.98 g cm-2)
As water is added the density drops
Car batteries contain concentrated sulfuric acid
As the battery is discharged, the concentration of the acid falls
By measuring the density of the acid the driver can check whether the battery is flat or not
Action as an oxidising agent
It behaves as an oxidising agent only when hot and concentrated:
Cu + 2H2SO4 CuSO4 + H2O + SO2 The sulphuric acid is reduced to sulphur dioxide
Action as a dehydrating agent
Concentrated sulphuric acid has a great affinity for water
(It is important when diluting the concentrated acid to add the acid to water and NEVER water to acid
) The reaction is highly exothermic
So great is its affinity for water that it can dehydrate compounds containing hydrogen and oxygen:
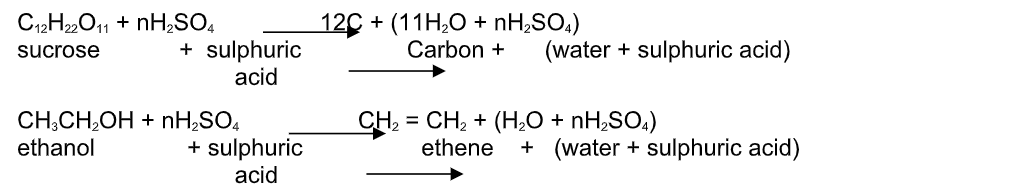
acid It is used for drying gases, especially SO2 and HCl, but cannot be used to dry a reducing gas such as H2S or an alkaline gas such as NH3
Action as a dehydrating agent
The properties of acids are due to the hydrogen ions in solution
Concentrated sulphuric acid contains molecules, rather than ions
Since it contains very few hydrogen ions it does not react significantly with metals and can safely be stored in steel containers
A piece of magnesium ribbon does not dissolve in concentrated sulphuric acid
Diluted with water, sulphuric acid behaves as a typical acid:
Industrial uses
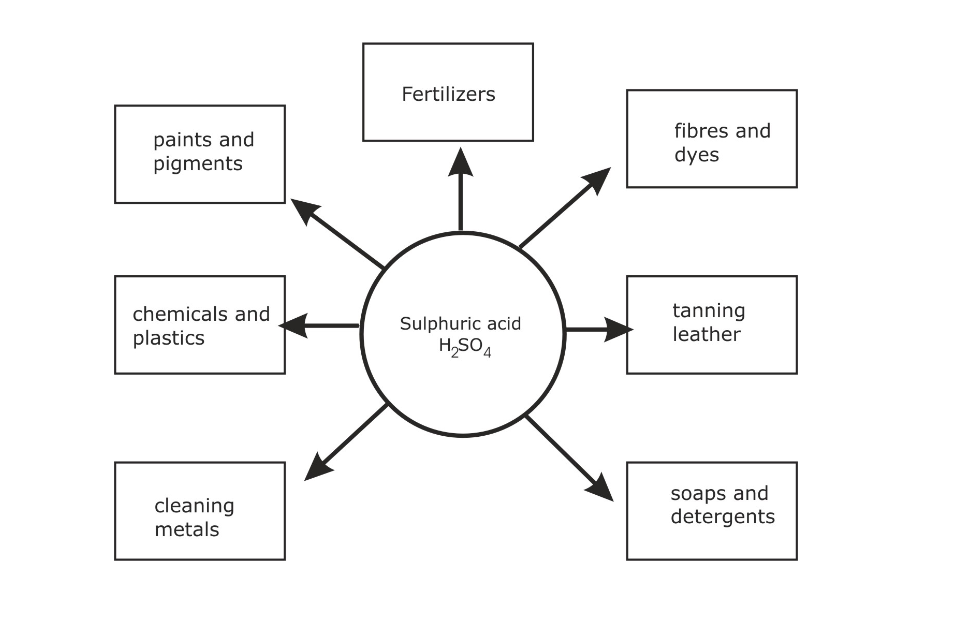
17.0.0 Chlorine and Its Compounds Chlorine
Halogen is elements in group (vii) of the periodic table
Chlorine is a halogen as well as fluorine, bromine, iodine and astatine
Chlorine has a symbol 35
5 Cl because it is made up of two isotopes 37 Cl and 35 Cl
It has an electronic arrangement of 2:8:7, hence justifying its position in group (vii)
Laboratory preparation
In order to convert hydrogen chloride to chlorine, it is necessary to remove hydrogen
Removal of hydrogen is oxidation
A powerful oxidizing agent such as manganese (IV) oxide converts hydrogen chloride (HCl) to chloride (Cl2) The most common laboratory method for preparation of Chlorine is to heat of Manganese Dioxide with concentrated Hydrochloric Acid
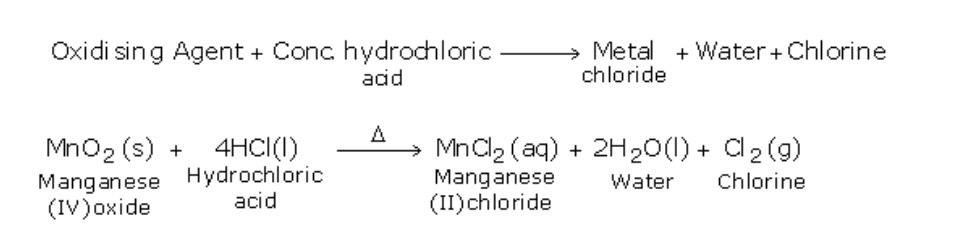
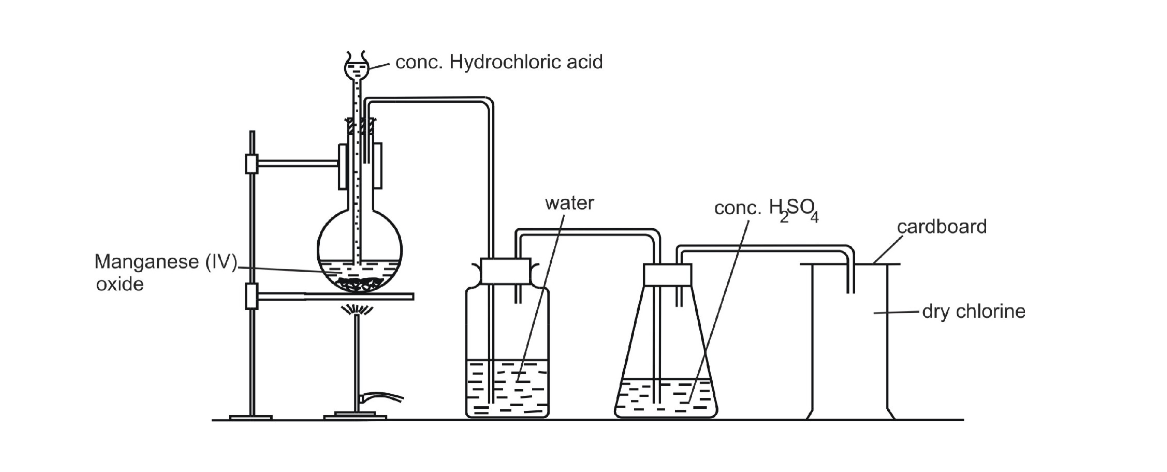
The gas is bubbled through water to remove any traces of hydrochloric gas that may be present and then it is dried by bubbling it through concentrated sulphuric acid
Chlorine may also be prepared by dropping cold concentrated Hydrochloric Acid on crystals of Potassium Permanganate
2 KMnO4 + 16 HCl 2 MnCl2 + 2 KCl + 8 H2O + 5 Cl2 The gas is also bubbled through water to remove any traces of Hydrochloric Acid gas that may be present and then it is dried by bubbling it through concentrated Sulphuric Acid
Manufacture of Chlorine
Membrane cell
Chlorine is manufactured industrially as a by-product in the manufacture of Caustic Soda by the electrolysis of brine
2 NaCl + 2 H2O Cl2 + H2 + 2 NaOH
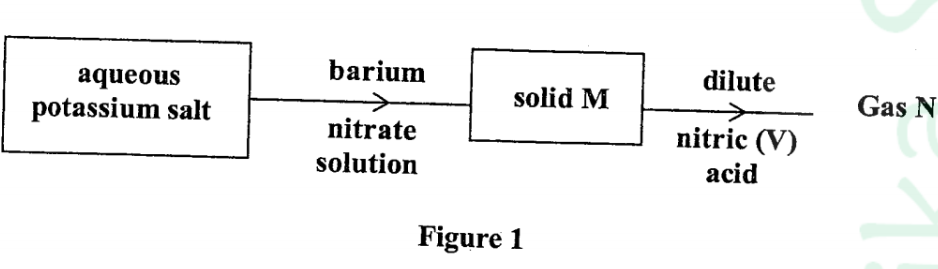
The membrane cell has titanium anode and a nickel cathode
Titanium is chosen because it is not attacked by chlorine
The anode and the cathode compartments are separated by an ion exchange membrane
The membrane is selective; it allows Na+ ions, H+ and OH- ions to flow but not Cl- ions
These ions cannot flow backwards, so products are kept separate and cannot react with each other At anode, the Cl- ions are discharged more readily than OH- ions because they are in higher concentration and are hence preferred
2 Cl- Cl2 + 2e- A pale green gas is seen coming off at the anode At cathode, it is the H+ ions that accept electrons, as sodium is more reactive than hydrogen 2 H+ + 2e- H2 Bubbles of hydrogen are seen at the cathode
The remaining ions of Na+ and OH- join up and come off as sodium hydroxide, NaOH
Products and uses
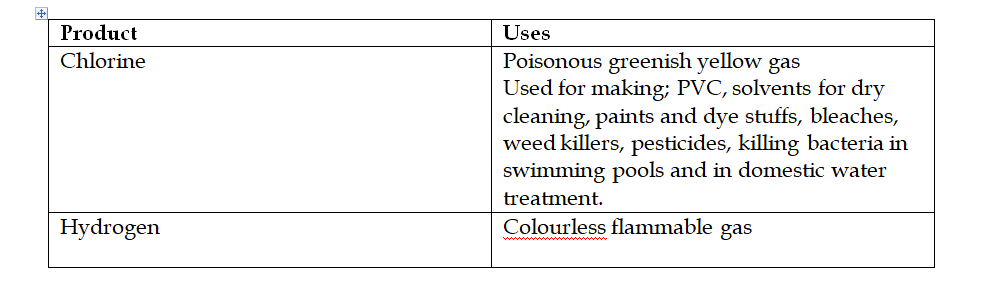
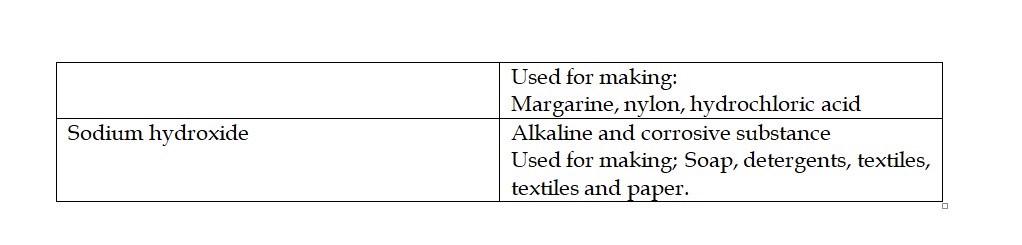
Properties of chlorine
Test for Chlorine Gas, Cl2(g)
1) Will turn moist litmus or universal indicator paper red, and then bleach it white
(2) Is green-yellow in colour
(3) Has a pungent choking smell and is poisonous
It is twice as dense as air
(4) Will put out a lit splint
Collecting Chlorine
Chlorine is denser than air and can be collected by downward delivery or using a gas syringe
Reactions
1.Chlorine is a highly reactive element, and undergoes reaction with a wide variety of other elements and compounds
2.Chlorine is a good bleaching agent, due to its oxidising properties
3.Chlorine is soluble in water (which solution is called Chlorine Water) and this loses its yellow colour on standing in sunlight, due to the formation of a mixture of Hypochlorous Acid and Hydrochloric Acid
Cl2 + H2O HOCl + HCl
4.Chlorine gas supports the vigorous combustion of many elements to form their chlorides
For example, Sulphur and Phosphorus burn in the gas
Cl2 + S SCl2 Cl2 + P PCl3 + PCl5
Bleaching Action
If chlorine is passed through water, it forms two acids, hydrochloric acid
Cl2 (g) + H2O (l) HCl (aq) + HOCl (aq)
Hypochlorous acid (the second acid) is the source of oxygen and is responsible for the bleaching of chlorine
HClO (aq) + Dye HCl (aq) + oxidized Dye (Coloured) (Colourless)
It is important to wash bleached clothes thoroughly to remove hydrochloric acid formed after the process
Reaction of Chlorine with Hydrogen
A mixture of Chlorine and Hydrogen explodes when exposed to sunlight to give Hydrogen Chloride
In the dark, no reaction occurs, so activation of the reaction by light energy is required
Hydrogen and chlorine gas also combine directly in presence of sunlight
A jar of hydrogen is inverted and placed on a jar containing chlorine in the sun
Soon hydrogen chloride is formed
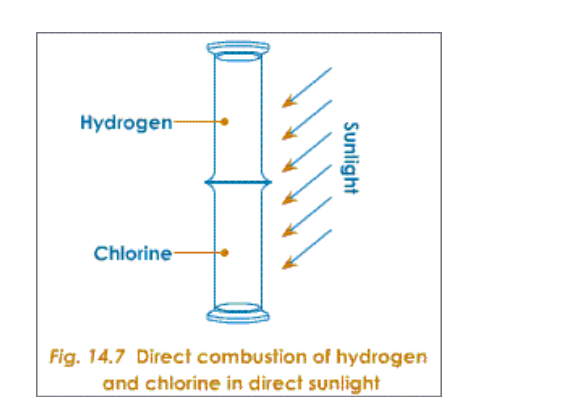
In diffused sunlight, the reaction slows down, and in the dark it is very slow
Cl2 + H2 2 HCl Hydrogen chloride is highly soluble in water
It dissolves to form hydrochloric acid
This reaction can be used to produce hydrochloric when the hydrogen chloride gas produced is dissolved in water as shown
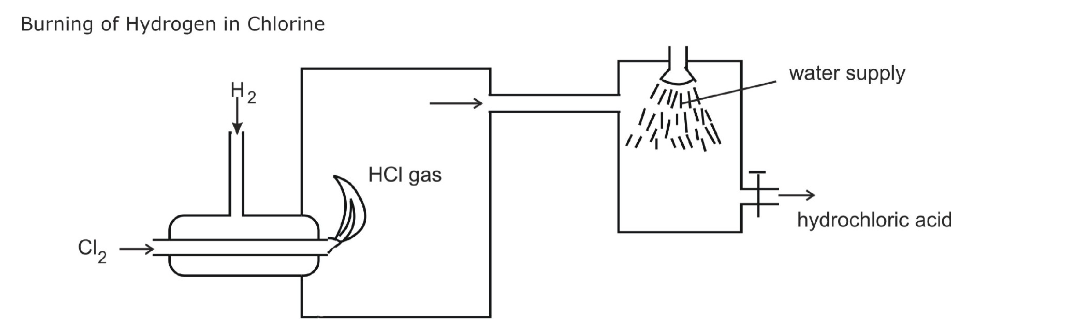
2. Reaction of Chlorine with Non-Metals
Chlorine combines directly with most non-metals (except with Nitrogen, Oxygen and Carbon, C)
3. Reaction of Chlorine with Metals
Thin foils of metals like sodium, copper, etc. when plunged into a jar of chlorine gas catch fire spontaneously to form their respective chlorides
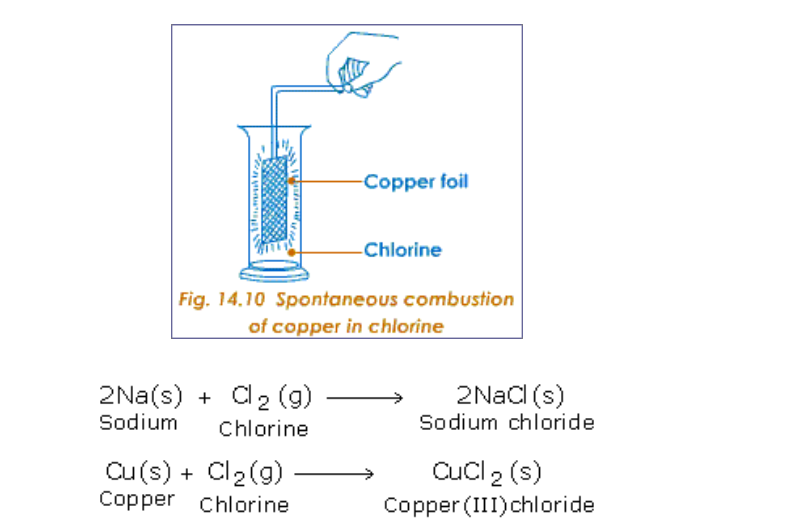
With yellow phosphorous
Yellow phosphorus first melts and then catches fire spontaneously when introduced into a jar of chlorine gas
It forms thick white fumes of phosphorus (III) chloride and phosphorus (V) chloride
Reaction with hydrogen sulphide
On passing chlorine and hydrogen sulphide through separate vents in a upright combustion tube hydrogen sulphide gets oxidised to hydrogen chloride and sulphur
Hydrogen chloride comes out through the middle tube
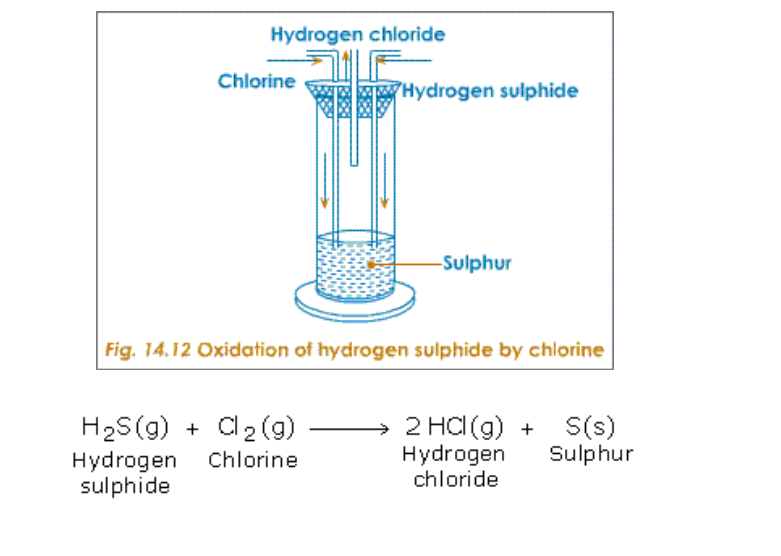
If chlorine is passed through a solution of hydrogen sulphide in water the solution turns turbid due to the formation of free sulphur
Reaction with aqueous sodium sulphite
When chlorine is passed through an aqueous solution of sodium sulphite it gets converted to sodium sulphate
Chlorine first reacts with the water to form nascent oxygen
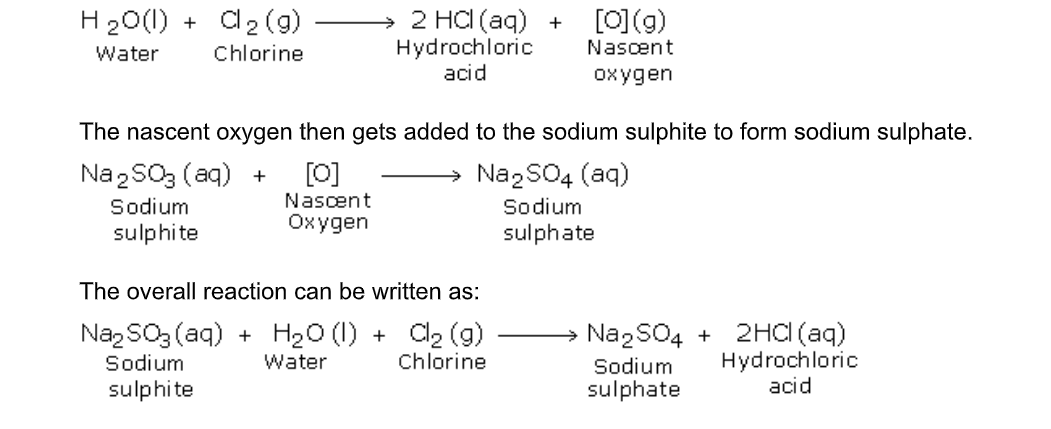
Displacement of the Halogens by Chlorine
Halogens are the most electronegative elements
Fluorine is the most electronegative, followed by chlorine then bromine and then iodine
The relative reactivity of the halogens, as described in group trends, can be shown by displacement reactions
These are similar to the metal displacement reactions
For example, Bromine gas bubbled through a solution of potassium iodide in water will displace (take the place of) the less reactive iodine, forming iodine and potassium bromide
Bromine + potassium iodide potassium bromide + iodine
Br2(g) + 2KI(aq) 2KBr(aq) + I2(s)
Similarly, chlorine will displace less reactive halogens
Chlorine will displace both bromine and iodine from the appropriate salt
This can be used in the extraction of bromine from sea water
Chlorine + potassium iodide potassium chloride + iodine
Cl2(g) + 2KI(aq) 2KCl(aq) + I2(s)
The equations can be written in terms of ions (called ionic equations)
For example, the last equation can be written as
Cl2(g) + 2I-(aq) 2Cl-(aq) + I2(s)
Potassium iodide, on the left, exists as potassium ions (K+) and iodide ions (I-) and potassium chloride, on the right, exists as potassium ions (K+) and chloride ions (Cl-)
Potassium ions (or other metal ions) can be left out of the ionic equation because they do not take part in the reaction
They are called ‘spectator ions’, as though they just sit back and watch!
Fluorine being the most electronegative halogen can displace all the other elements of this group (chlorine, bromine and iodine) from the aqueous solution of their salts
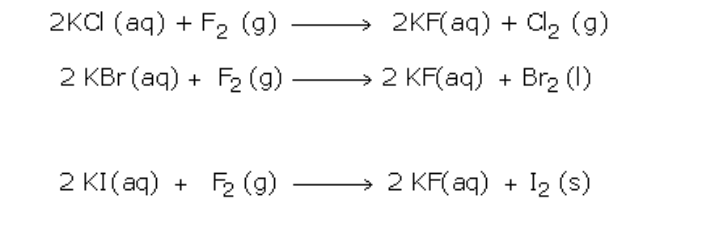
Chlorine can displace bromine and iodine from bromides and iodides respectively
However, it cannot displace fluorine from fluorides
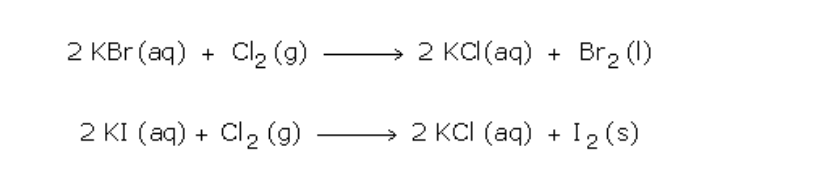
Bromine can displace iodine from iodides
But it cannot displace chlorine or fluorine from chlorides or fluorides respectively
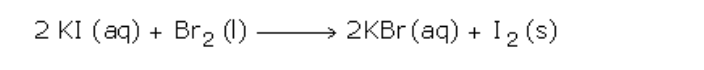
Iodine being the least electronegative of the halogens cannot displace any other halogen from their respective halides
Summary of Displacement Reactions
Bleaching Action of Chlorine
Chlorine bleaches (removes the color) organic colors by the process of oxidation in presence of moisture
The bleaching action takes place in few steps:
a) Chlorine first dissolves in water to give a mixture of hydrochloric acid and hypochlorous acid
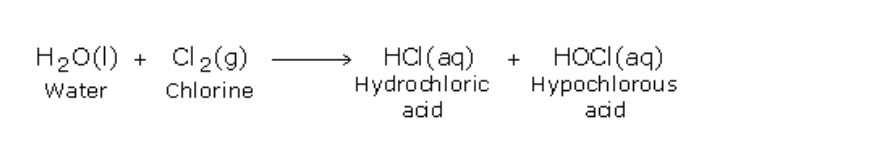
b) As mentioned earlier hypochlorous acid is very unstable and decomposes to give hydrochloric acid and nascent oxygen
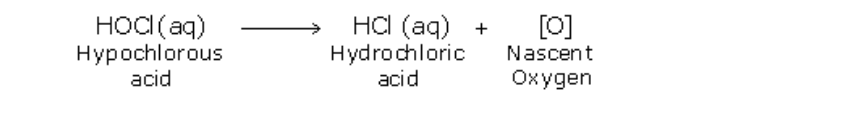
c) The nascent oxygen oxidises the coloring matter to colorless matter thereby bleaching them
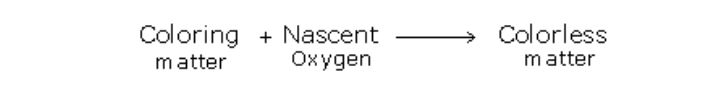
Bleaching by chlorine is permanent
Chlorine bleaches cotton fabrics, wood pulp litmus etc
However, it is not used to bleach delicate articles such as silk, wool etc
, as it is a strong bleaching and oxidizing agent
This dual action will damage the base material
You must have noticed that in the very first stage, presence of water is essential to produce hypochlorous acid
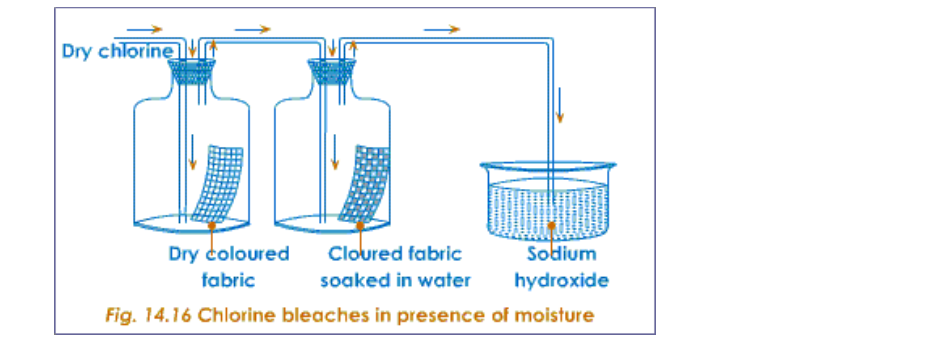
The dry coloured fabric does not bleach
The coloured fabric soaked in water is bleached
This shows that chlorine only bleaches in the presence of water
If water is absent no bleaching can take place
Dry chlorine therefore does not bleach
Tests for Chlorine
1. Chlorine is a greenish yellow gas with a pungent and irritating odor
2. It turns wet blue litmus paper red and then bleaches it
3. Colored petals and the leaves of plants can be bleached by it
4. Chlorine turns wet starch potassium iodide paper blue by displacing the iodine from the potassium iodide, and causing iodine to turn the starch blue
Oxidizing Reaction of Chlorine
Chlorine is a strong oxidising agent
Chlorine oxidises Iron (II) Chloride, FeCl2, to the salt containing Iron in the higher oxidation state Iron (III) Chloride, FeCl3
This is possible because Iron has a variable valency
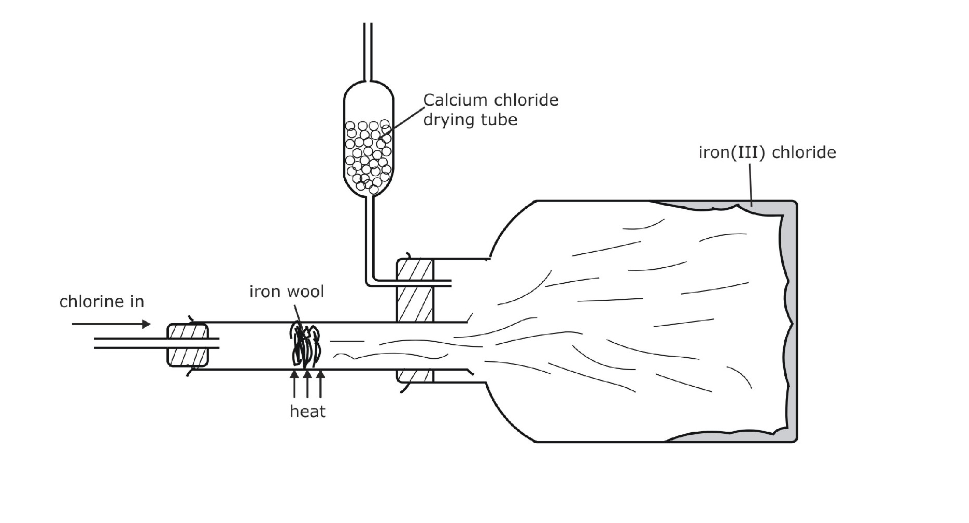
2 FeCl2 + Cl2 2 FeCl3 Chlorine displaces the less electronegative Bromine and Iodine from their respective salts
Cl2 + 2 KBr 2 KCl + Br2 Chlorine removes Hydrogen from the hydrides of non-metals, forming Hydrogen Chloride, and leaving the non-metal element
Cl2 + H2S 2 HCl + S
Affinity for Hydrogen
Chlorine combines with free hydrogen to form hydrogen chloride
It can also react with the hydrogen present in other compounds such as water, ammonia, hydrocarbon, hydrogen sulphide etc
With water
Chlorine dissolves in water to form chlorine water
It slowly reacts with the water to form a mixture of hydrochloric acid and hypochlorous acid
Hypochlorous acid is very unstable and in presence of sunlight it decomposes to give hydrochloric acid and a nascent oxygen atom
Two such nascent oxygen atoms combine to form a molecule of oxygen
So if a solution of chlorine in water is exposed to sunlight as shown in figure 14
14, oxygen is formed
With ammonia
Depending on which of the two gases is in excess, chlorine reacts with ammonia in two ways
(a) When ammonia is in excess the final products are ammonium chloride and nitrogen
Hydrogen chloride thus produced reacts with excess of ammonia to produce ammonium chloride
The overall reaction can be written as:
(b) When chlorine is in excess the final product is an oily explosive liquid called nitrogen trichloride
With alkalis
Alkalis, at different temperatures and at different levels of concentration, behave differently with chlorine
(i) With cold dilute alkalis
Chlorine reacts with cold dilute alkalis to form their respective chlorides, hypochlorites and water
(ii) With hot concentrated alkalis
Chlorine reacts with hot concentrated alkalis to form their respective chlorides, chlorates and water
Uses
Chlorine is used
Hydrogen Chloride Hydrogen chloride is an hydrogen compound of Chlorine
Chlorine is a highly reactive element and is mainly found in combined state
Its most important source is sodium chloride which is mainly found in large underground deposits, sea and lake such as lake Magadi
Sodium chloride is the main source of chlorine which is used to make hydrogen chloride
Preparation of hydrogen chloride
Hydrogen Chloride may be prepared in the laboratory by heating Concentrated Sulphuric Acid, with Sodium Chloride
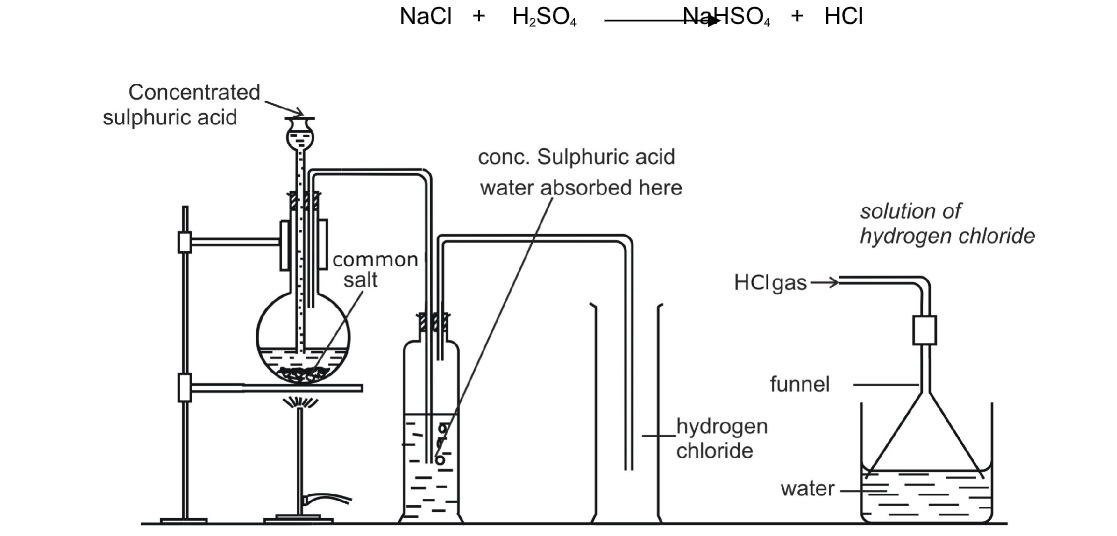
Preparing and making a solution of hydrogen chloride
It is prepared industrially by the combustion of Hydrogen, H2, in Chlorine, Cl2
H2 + Cl2 -> 2 HCl
Other way of producing chlorine
Chlorine, removes Hydrogen, from the hydrides of non-metals, forming Hydrogen Chloride, and leaving the non-metal element
Cl2 + H2S 2 HCl + S
When Chlorine Water, (i.e a solution of Chlorine gas, in Water) in a flask inverted in a basin of the same liquid is exposed to bright sunlight, the Chlorine is decomposed and a solution of Hypochlorous Acid remains
H2O + Cl2 HCl + HClO
The Hypochlorous Acid, is not very stable and the solution readily decomposes, especially when exposed to sunlight, yielding Oxygen, 2 HClO 2 HCl + O2 Chlorine is soluble in water (which solution is called Chlorine Water) and this loses its yellow colour on standing in sunlight, due to the formation of a mixture of Hypochlorous Acid, and Hydrochloric Acid
Cl2 + H2O HOCl + HCl
Properties of Hydrogen Chloride
Hydrogen chloride in solution
Hydrogen chloride is a colourless fuming gas
The polar covalent gas Hydrogen Chloride is very soluble in Water
In aqueous solution, the molecule exists in ionic form, as the positively charged Hydrogen Ion, H+, and the negatively charged Chloride Ion, Cl-
HCl + aq H (+) + Cl (-) Hydrogen Hydrogen Chloride Chloride Ion Ion Its solution in water turns blue litmus paper red
Hydrogen chloride has no effect on dry litmus paper as no ions are present in dry gas
The importance of water
The gas hydrogen chloride is made up of covalently bonded molecules
If the gas is dissolved in an organic solvent, such as methylbenzene, it does not show any of the properties of an acid
Dissolving hydrogen chloride in water and methylbenzene
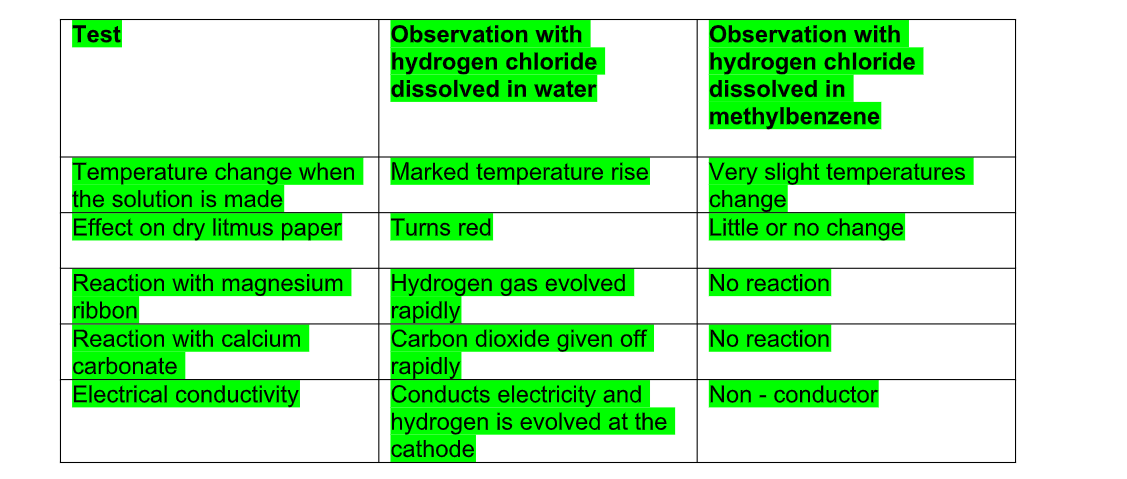
For example, it does not conduct electricity or turn a piece of blue litmus paper red
However, when the gas is dissolved in water, a strongly acidic solution is produced
The acidic oxides of sulphur, phosphorus and carbon are the similar
They are covalent molecules when pure, but show acidic properties only when dissolved in water
Reaction with Group 1 Alkali Metals
Alkali metals burn very exothermically and vigorously when heated in chlorine to form colourless crystalline ionic salts e.g NaCl or Na+Cl-
This is a very expensive way to make salt! It’s much cheaper to produce it by evaporating sea water!
E.g sodium + chlorine sodium chloride
2Na(s) + Cl2(g) 2NaCl(s)
The sodium chloride is soluble in water to give a neutral solution pH 7, universal indicator is green
The salt is a typical ionic compound i.e a brittle solid with a high melting point
Similarly potassium and bromine form potassium bromide KBr, or lithium and iodine form lithium iodide LiI
Again note the group formula pattern
Hydrochloric acid reacts with metals above hydrogen in the reactivity series
All of these metals react with hydrochloric acid liberating hydrogen which puts out a burning splint with a pop sound
Zn(s) + 2HCl (aq) -> ZnCl2 (aq) + H2 (g)
This is a common reaction for preparing hydrogen gas in the school laboratory
The hydrogen can then be collected over water
The salts produced can be obtained from the solution by filtration to obtain the filtrate which then transferred onto an evaporation dish for evaporation and crystallization
No hydrogen is liberated when the acid is reacted with lead, copper and mercury
Despite the fact that lead is above hydrogen in the reactivity series, the hydrogen chloride is not a strong enough oxidizing agent to liberate hydrogen
Reaction with bases
Hydrochloric acid is a strong acid as it is well ionized in solution
It neutralizes bases and alkalis forming salts and water
KOH (aq) + HCl (aq) -> KCl (aq) + H2O (l)
CuO(s) + HCl (aq) -> CuCl2 (aq) + H2O (l) FeO(s) + 2 HCl (aq) -> FeCl2 (aq) + H2O (l)
Reaction with carbonates
Hydrogen chloride reacts with carbonates and hydrogen carbonates producing carbon dioxide, salt and water
Carbon dioxide turns lime water milky
CaCO3 (aq) + 2HCl (aq) CaCl2 (aq) + CO2 (g)+H2O (l)
Ionic equation CO3/sub>2- (aq) + 2H+ (aq) CO2(g) + H2O (l)
NaHCO3 (aq) + HCl (aq) NaCl (aq) + CO2 (aq) + H2O (l)
Ionic equation
HCO3-(aq) + H+ (aq) CO2(g) + H2O(l)
Reaction with hydrogen H2
Halogens readily combine with hydrogen to form the hydrogen halides which are colourless gaseous covalent molecules
E.g hydrogen + chlorine hydrogen chloride
H2 (g) + Cl2 (g) -> 2HCl (g)
The hydrogen halides dissolve in water to form very strong acids with solutions of pH1 e.g hydrogen chloride forms hydrochloric acid in water HCl(aq) or H+Cl-(aq) because they are fully ionised in aqueous solution even though the original hydrogen halides were covalent!
An acid is a substance that forms H+ ions in water
Bromine forms hydrogen bromide gas HBr (g), which dissolved in water forms hydrobromic acid HBr (aq)
Iodine forms hydrogen iodide gas HI (g), which dissolved in water forms hydriodic acid HI (aq)
Note the group formula pattern
Oxidation of hydrochloric acid
MnO2 + 4 HCl MnCl2 + 2 H2O + Cl2 The gas is bubbled through water to remove any traces of hydrochloric gas that may be present and then it is dried by bubbling it through concentrated sulphuric acid
Chlorine may also be prepared by dropping cold concentrated Hydrochloric Acid, on Potassium Permanganate
2KMnO4 + 16HCl 2MnCl2 + 2KCl + 8H2O + 5Cl2Summary
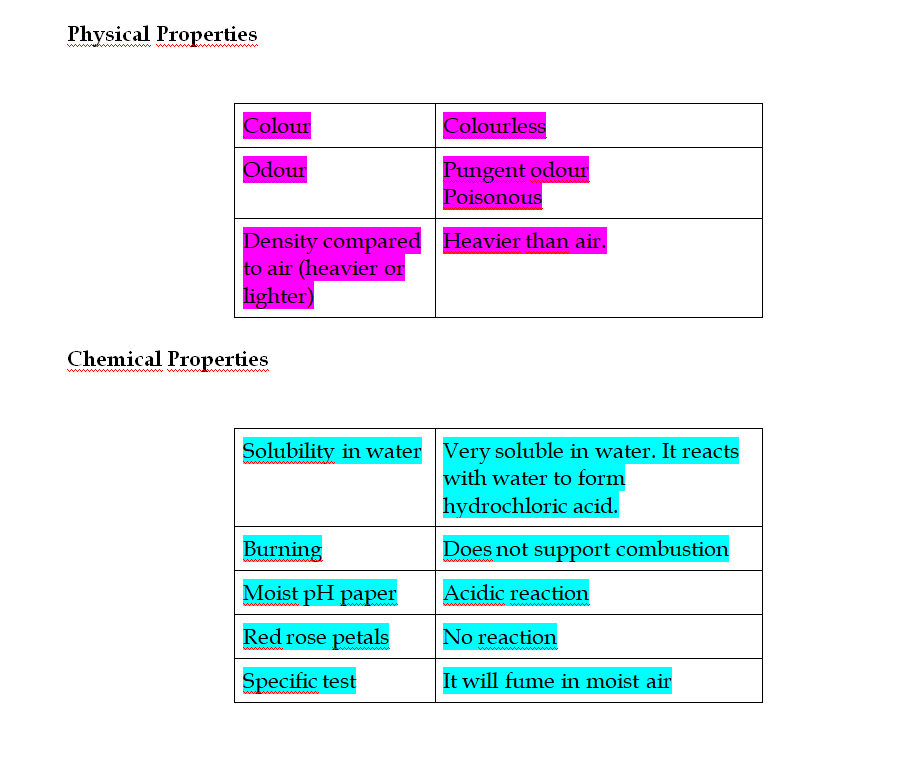
Defination of Common Terms in Chemistry
Word Definition:
Acid :A corrosive substance which has a pH lower than 7
Acidity is caused by a high concentration of hydrogen ions
Acid rain: Acid rain is caused when sulphur dioxide – released by the burning of coil and oil – dissolves in rainwater to form sulphuric acid
Activation energy: The minimum energy required for a collision between particles, in order for a reaction to occur
Addition polymer: Addition polymers are made when monomers (simple molecules) add together across a double bond
Addition reaction: A reaction in which a small molecule adds on across a double bond
Alkali: A base, which is soluble in water
Alkali metals The alkali metals are the elements in Group 1 of the periodic table
They have one electron in the outer shell
Alkanes: Alkanes are saturated hydrocarbons
This means that each carbon atom has four bonds to other atoms
Alkenes: Alkenes are unsaturated hydrocarbons with a double bond between the carbon atoms
Allotropes: Allotropes are structurally different forms of an element
They differ in the way the atoms bond with each other and arrange themselves into a structure
Because of their different structures, allotropes have different physical and chemical properties
Alloy: An alloy is a mof two or more elements, at least one of which is a metal
Anode: An anode is the electrode (electrical conductor) attached to the positive terminal of a battery
Atom: All elements are made of atoms
An atom consists of a nucleus containing protons and neutrons, surrounded by electrons
Atomic number: The atomic number (Z) of an element is the number of protons in the nucleus of the atom
Bases: Substances with a pH higher than 7, and which have a high concentration of hydroxyl ions
Bases react with acids to form a salt and water (called neutralisation)
Metal hydroxides, oxides and carbonates are all bases
Biocatalyst :A biocatalyst is an enzyme or microorganism that activates or speeds up a biochemical reaction
Boiling point: The temperature at which a liquid changes its state to gas
Brittle If something is brittle it is easily broken
Catalyst: A catalyst changes the rate of a chemical reaction without being changed by the reaction itself
Catalytic converter: A device in internal combustion engines which catalyses reactions converting harmful exhaust gases such as carbon monoxide into normal atmospheric gases
Cathode: A cathode is the electrode (electrical conductor) attached to the negative terminal of a battery
Chemical change: A chemical change involves new substances being formed and is very difficult to reverse
Combustion: Combustion is the process of burning by fire
Compound: A compound is a substance formed by the chemical union (involving bond formation) of two or more elements
Condensation: Condensation is a change of state in which gas becomes liquid by cooling
Conduct To allow electricity, heat or other energy forms to pass through
Conductor: An electrical conductor is a material which allows an electrical current to pass easily
It has a low resistance
A thermal conductor allows thermal energy to be transferred through it easily
Corrode: To deteriorate (get weaker) due to the action of water, air, or an acid
Covalent bond: A covalent bond between atoms forms when atoms share electrons to achieve a full outer shell of electrons
Covalent compounds: A covalent compound is a compound of neutral atoms in which the atoms are held together by covalent bonds
Covalent bonds between atoms form when atoms share electrons to achieve a full outer shell of electrons
Cracking: Cracking is the breaking down of large hydrocarbon molecules into smaller, more useful hydrocarbon molecules by vapourizing them and passing them over a hot catalyst
Crude oil : Crude oil is formed from the remains of small animals and plants that died and fell to the bottom of the sea
Their remains were covered by mud
As the sediment was buried by more sediment, it started to change into rock as the temperature and pressure increased
The plant animal remains were “cooked” by this process and changed into crude oil
Decomposition: A reaction in which substances are broken down, by heat, electrolysis or a catalyst
Denatured: If a protein is denatured, its structure and function is altered
This can be caused by heat, altered pH or by chemical agents
Displacement reactions: Displacement reactions happen when a more-reactive element replaces a less-reactive element in a compound
Distillation: Distillation is when we make a liquid evaporate and then condense the vapour back to a purer liquid
Double bond :A double bond is a covalent bond resulting from the sharing of four electrons (two pairs) between two atoms
Ductile: If a material is ductile it is capable of being drawn into thin sheets or wires without breaking
Electrode: Electrodes are conductors used to establish electrical contact with a circuit
The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode
The electrode attached to the positive terminal of a battery is the positive electrode, or anode
Electrolysis: Electrolysis is the decomposition (separation or break-down) of a compound using an electric current
Electrolyte An electrolyte is a substance which in solution will conduct an electric current
Electron: An electron is a very small negatively charged particle found in an atom in the space surrounding the nucleus
Electrostatic: An electrostatic force is generated by differences in electric charge (i.e positive and negative) between two particles
It can also refer to electricity at rest
Element All atoms of an element have the same atomic number, the same number of protons and electrons and so the same chemical properties
Endothermic: In an endothermic reaction, energy is taken in from the surroundings
The surroundings then have less energy than they started with, so the temperature falls
Equilibrium: If the rate of the forward reaction and the rate of the back reaction in a reversible reaction are equal, the reaction is in equilibrium
Eutrophication: Eutrophication is the enrichment of a water body with nutrients – such as nitrates – which results in excessive growth of algae and other aquatic plants, leading to depletion of oxygen
Evaporation: Evaporation is a change in state in which a liquid becomes a gas (vapour); molecules near the surface of a liquid may leave the liquid to become a vapour
Exothermic: Heat energy is released in an exothermic reaction
We know this because the surroundings get warm
Filtrate: Filtrate is fluid that has passed through a filter
Formula: A formula is a combination of symbols that indicates the chemical composition of a substance
Fossil: Fossils are the remains of animals and plants from a past geological age, preserved in the Earth’s crust
Fossil fuels: Fossil fuels have been created over millions of years by the decay and compression of living things, particularly plants
Coal, gas and oil are fossil fuels
Fractional distillation: In fractional distillation a mixture of several substances, such as crude oil, is distilled and the evaporated components are collected as they condense at different temperatures
Groups: The groups of elements in the periodic table are the elements which have the same number of electrons in their outer shells and so have similar chemical properties
A group of elements all lie in same column in the periodic table
Halogens: The halogens are the elements in Group VII of the periodic table
They have seven electrons in the outer shell
Hydrocarbons: Hydrocarbons are a group of compounds, which contain the elements hydrogen and carbon
Ion :An ion is a charged particle formed by loss or gain of electrons
When atoms lose an electron they become a positive ion
When they gain an electron they become a negative ion
Ionic bond: An ionic bond forms between two atoms when an electron is transferred from one atom to the other, forming a positive-negative ion pair
Ionic compound: An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom that has lost an electron)
The ions swap electrons to achieve a full outer shell
Isotopes: Atoms of the same element that have different numbers of neutrons are called isotopes
Lattice: A lattice is a regular grid-like arrangement of atoms in a material
Liquefy: To liquefy means to become liquid, for example through heating a solid or cooling a gas
Lubricant: A substance used to reduce the friction between two solid surfaces
Mass: Mass is a measure of the amount of material in an object
It is measured in grams (g)
Mass number: The mass number (A) of an element is the number of protons plus the number of neutrons in the nucleus of the atom
Molecular compound: A molecular compound is made up of at least two different elements, which share electrons to form covalent bonds
Molecule: A molecule is a collection of two or more atoms held together by chemical bonds
It is the smallest part of a substance that displays the properties of the substance
Molten: Molten means reduced to liquid form by heating
It is mainly used to describe rock, glass or metal
Monomer: A monomer is a simple molecule
Natural gas: Natural gas is a fossil fuel formed from decaying plant and animal material
Neutralisation: Neutralisation is the reaction between an acid and a base to form a salt plus water
Neutron: A neutron is a particle that is found in the nucleus of an atom, has a mass approximately equal to that of a proton, and has no electric charge
Noble gases: The noble gases are the elements in Group 0/Group VIII of the periodic table
They have a full outer shell of electrons and so are unreactive
Nucleus: Found at the centre of an atom, the nucleus contains protons and neutrons
Ore: An ore is a rock containing enough quantities of a mineral that it is profitable to extract it
Oxidation: Oxidation is a reaction in which oxygen combines with a substance
Oxidation also means a loss of electrons
Periods: The periods of elements in the periodic table are the elements in which the same outer shell is being filled up
A period of elements in the periodic table all lie in the same row
Polymer: A polymer is a large molecule formed from many identical smaller molecules (monomers)
Polymerise: Polymerisation is the reaction in which many identical monomers are joined together to make a polymer
Product: A product is a substance formed in a chemical reaction
Protons: A proton is a small particle with a positive charge found in the nucleus of the atom
Radioactive: A radioactive isotope gives off (or is capable of giving off) radiant energy in the form of particles or rays by the spontaneous disintegration of the nucleus
Reactant: A reactant is a substance put together with another substance/substances to undergo a chemical reaction
Reactant: A reactant is a substance put together with another substance/substances to undergo a chemical reaction
Re-crystallisation: When rocks are heated and put under pressure new crystals can form
These are often elongated in the direction of least pressure
Redox: reaction Oxidation and reduction always take place together
The combined reaction is called a redox reaction
Reduction: Reduction is a reaction in which oxygen is removed from a substance
Reduction also means a gain in electrons
Relative atomic mass: The relative atomic mass is the number of times heavier an atom is compared to one twelfth of a carbon-12 atom
Relative mass: The relative mass is the number of times heavier a particle is, compared to another
Reversible reactions :Reversible reactions are chemical reactions, which can go both ways
The direction of the reaction depends on the condition of the reactants
Salt: A compound formed by neutralisation of an acid by a base (e.g a metal oxide) – the result of hydrogen atoms in the acid being replaced by metal atoms or positive ions
Sodium chloride: – common salt – is one such compound
Saturated: Filled to capacity
In a saturated hydrocarbon there are no more available bonds
Slag :Slag is the non-metallic by-product resulting from the extraction of metals such as iron from their ores
Solute: A solute is the material that dissolves in a solvent to form a solution
Solution: A solution is the mixture formed when a solute dissolves in a solvent
Solvent: A solvent is the liquid in which the solute dissolves to form a solution
Stable: Atoms are stable if their outer shell contains its maximum number of electrons
Sterilize: To sterilize means to make something free from microorganisms such as bacteria
Thermal decomposition: A reaction in which substances are broken down by heat
Unsaturated: An unsaturated compound contains at least one double or triple bond
Vapour: Vapour is a cloud of liquid particles
Steam is water vapour
Vapourized: Turned (generally through heating) into vapour.Vapour is a cloud of liquid particles
Steam is water vapour
KCSE Revision Notes Form 1 – Form 4 All Subjects
Basic Chemistry Interview Questions and Answers Pdf Basic Chemistry Questions and Answers Basic Chemistry Questions and Answers Pdf Chemistry Form 1 Notes Chemistry Form 1 Notes Free Download Chemistry Form 1 Notes Pdf Chemistry Form 1 Notes Pdf Download Chemistry Form 2 Notes Chemistry Form 2 Questions and Answers Chemistry Form 2 Salts Chemistry Form 2 Structure and Bonding Chemistry Form 3 Notes Chemistry Form 3 Notes Pdf Chemistry Form 4 Exercise Chemistry Form 4 Notes Chemistry Form 4 Notes Chapter 1 Chemistry Form 4 Notes Chapter 2 Chemistry Form 4 Notes Chapter 3 Chemistry Form 4 Notes Download Chemistry Form 4 Notes Pdf Chemistry Form One Book Pdf Chemistry Form One Notes Pdf Chemistry Form One Pdf Chemistry Multiple Choice Questions and Answers Pdf Chemistry Objective Questions for Competitive Exams Chemistry Question and Answer With Explanation Chemistry Questions and Answers Chemistry Questions and Answers for High School Chemistry Questions and Answers Pdf Chemistry Questions and Answers Pdf for Class 12 Chemistry Quiz Questions and Answers for Class 12 Chemistry Quiz Questions and Answers Multiple Choice Chemistry Spm Notes Download Download Chemistry Form 2 Notes Download Klb Chemistry Book 3 Form 3 Chemistry Questions and Answers Form 3 Chemistry Questions and Answers Pdf Form Three Chemistry Book Pdf Hard Chemistry Questions and Answers High School Chemistry Questions and Answers Pdf Inorganic Chemistry Multiple Choice Questions With Answers Pdf Inorganic Chemistry Questions and Answers Pdf Interesting Chemistry Questions Klb Chemistry Book 1 Download Klb Chemistry Book 1 Pdf Klb Chemistry Book 2 Notes Klb Chemistry Book 2 Notes Pdf Klb Chemistry Book 3 Notes Klb Chemistry Form 2 Notes Klb Chemistry Form 2 Pdf Klb Chemistry Form 3 Pdf Spm Chemistry Revision Notes Tricky Chemistry Questions With Answers “Pdf” Revision Questions Chemistry Form 2 “Pdf” Revision Questions Chemistry Form 3 “Pdf” Revision Questions Chemistry Form 4 “Pdf” Revision Questions Chemistry Form Four “Pdf” Revision Questions Chemistry Form One “Pdf” Revision Questions Chemistry Form Three “Pdf” Revision Questions Chemistry Form Two 1 a a KCSE Past Papers 10th Grade Chemistry Questions and Answers 10th Grade Chemistry Test 11th Ncert Chemistry 12th Class Chemistry Book Free Download 2014 KCSE Marking Schemes 2014 Pdf KCSE Past Papers 2015 2015 Chemistry Essay Questions and Answers Form 4 2016 KCSE Papers 2016 KCSE Prediction Questions 2017 Chemistry Hsc Answers 2017 KCSE Prediction Questions 2018 Chemistry KCSE Leakage 2018 Chemistry KCSE Questions 2018 KCSE Busineness Studies 2018 KCSE Exam 2018 KCSE Leakage 2018 KCSE Prediction Questions 2018 KCSE Questions 2019 Chemistry KCSE Leakage 2019 Chemistry KCSE Questions 2019 KCSE Leakage 2019 KCSE Questions 9th Grade Chemistry Study Guide A a a Chemistry Notes a a a Chemistry Notes! a a a ChemistryNotes! A a KCSE Past Papers A Biblical View of Social Justice A Level Chemistry Biological Molecules Questions A Level Chemistry Exam Questions by Topic A Level Chemistry Notes Edexcel A Level Chemistry Notes Xtremepapers A Level Chemistry Past Papers A Level Chemistry Questions and Answers a Level Chemistry Questions and Answers A Level Chemistry Questions and Answers (Pdf) A Level Chemistry Questions and Answers Pdf A Level Chemistry Questions by Topic Kidney Questions With Markschemes A Level Chemistry Revision A Level Chemistry Revision Edexcel A Level Chemistry Revision Guide A Level Chemistry Revision Notes A Level Chemistry Revision Notes Pdf A Level Chemistry Textbook Pdf A Level Chemistry Year 1 / as Aqa Exam Questions by Topic A Level Edexcel Notes a* Chemistry aa Chemistry Form 3 Questions and Answers Advance KCSE Past Papers Advance-africa.com KCSE Rev Quiz Advantages and Disadvantages. All Chemistry Essays All Chemistry Notes for Senior Two All KCSE Past Papers Chemistry With Making Schemes All Marking Schemes Questions and Answers All Past K.c.s.e Questions With Answers Alliance Mocks 2017 Ap Bio Quizzes Ap Chemistry 1 Textbook Pdf Ap Chemistry Essay Questions and Answers Are Sourced From KNEC. As Level Chemistry Notes Atika Chemistry Notes Atika School Chemistry Notes B/s Book 2 Notes Basic Chemistry Books Pdf basic Chemistry Interview Questions and Answers Pdf Basic Chemistry Interview Questions and Answers Pdf Basic Chemistry Pdf Basic Chemistry Questions and Answers Basic Chemistry Questions and Answers Pdf Bbc Bitesize Chemistry Ks3 Bihar Board Chemistry Objective Answer 2017 Bihar Board Chemistry Objective Answer 2018 Bio Answers Bio Quesions Chemistry 0478 Chemistry 101 Chemistry 12th Chemistry 12th Class Notes Pdf Chemistry 2019 Syllabus Chemistry All KCSE Short Notes Chemistry Answers Chemistry Answers Online Free Chemistry Answers Quizlet Chemistry Bk 2 Notes Chemistry Book 1 Chemistry Book 1 Notes Chemistry Book 2 Chemistry Book 2 Notes Chemistry Book 3 Chemistry Book 3 KLB Chemistry Book 3 Notes Chemistry Book 4 Chemistry Book 4 Notes Chemistry Book 4 Pdf Chemistry Book for Class 11 Chemistry Book Four Chemistry Book Four Notes Chemistry Book One Chemistry Book One Notes Chemistry Book Pdf Free Download Chemistry Book Three Chemistry Book Three Notes Chemistry Book Three Pdf Chemistry Book Two Chemistry Book Two Notes Chemistry Books Form Three Chemistry Bowl Chemistry Study Guide Chemistry Bowl Questions Chemistry Chemistry Bowl Questions Earth Chemistry Chemistry Bowl Questions Math Chemistry Bowl Questions Middle School Chemistry Brekthrough Form Two Notes Chemistry Class 12 Ncert Solutions Chemistry Class 12 Pdf Chemistry Communication Syllabus Chemistry Diagram Software Chemistry Diagrams for Class 11 Chemistry Diagrams for Class 12 Chemistry Diagrams for Class 9 Chemistry Diagrams for Class-10 Chemistry Diagrams in Form 1 Chemistry Diagrams in Form 2 Chemistry Diagrams in Form 3 Chemistry Diagrams in Form 4 Chemistry Diagrams Pdf Chemistry Diagrams to Label Chemistry Essay Questions and Answers Chemistry Essay Questions and Answers 2018 Chemistry Essay Questions and Answers Form 1 Chemistry Essay Questions and Answers Form 2 Chemistry Essay Questions and Answers Form 3 Chemistry Essay Questions and Answers Form 4 Chemistry Essay Questions and Answers Form 4 Pdf Chemistry Essay Questions and Answers Pdf Chemistry Essay Revision Q Chemistry Essays and Answers Chemistry Essays Form One to Form Four Chemistry Essays Form One to Form Three Chemistry Essays KCSE Chemistry Essays Pdf Chemistry Exam 1 Multiple Choice Chemistry Exam 2 Advance Chemistry Exam 2 Test Chemistry Exam 2016 Chemistry Exam Form Four Chemistry Exam Form One Chemistry Exam Form Three Chemistry Exam Form Two Chemistry Exam Practice Test Chemistry Exam Questions Chemistry Exam Questions and Answers Chemistry Exam Questions and Answers Pdf Chemistry Exam Study Guide Chemistry Exams Chemistry Excretion Notes Chemistry Exercise Form 4 With Answers Chemistry Final Exam Answer Key Chemistry Final Exam Answer Key 2016 Chemistry Final Exam Answer Key 2017 Chemistry Final Exam Answers 2018 Chemistry Final Exam Answers 2019 Chemistry Final Exam Questions and Answers Chemistry Fom 1 Notes Chemistry Fom 2 Notes Chemistry Fom 3 Notes Chemistry Fom 4 Notes Chemistry Form 1 Chemistry Form 1 & 2 and Answers Chemistry Form 1 and 2 Essays Chemistry Form 1 and 2 Essays Questions and Answers Chemistry Form 1 Chapter 1 Chemistry Form 1 Diagrams Chemistry Form 1 Exams Chemistry Form 1 Mid Year Exam Chemistry Form 1 Notes Chemistry Form 1 Notes and Questions Chemistry Form 1 Notes Download Chemistry Form 1 Notes Free Download Chemistry Form 1 Notes GCSE Chemistry Form 1 Notes KCSE-kcse Chemistry Form 1 Notes Pdf Chemistry Form 1 Notes Pdf Download Chemistry Form 1 Past Papers Chemistry Form 1 Pdf Chemistry Form 1 Pressure Chemistry Form 1 Question Papers Chemistry Form 1 Questions Chemistry Form 1 Questions and Answers Chemistry Form 1 Questions and Answers Pdf Chemistry Form 1 Quiz Chemistry Form 1 Revision Questions Chemistry Form 1 Summary Notes Chemistry Form 1 Syllabus Chemistry Form 1 Work Chemistry Form 1-4 Notes Chemistry Form 2 Chemistry Form 2 Chapter 1 Chemistry Form 2 Chapter 2 Chemistry Form 2 Diagrams Chemistry Form 2 Exam Paper 2014 Chemistry Form 2 Exams Chemistry Form 2 Notes Chemistry Form 2 Notes and Questions Chemistry Form 2 Notes GCSE Chemistry Form 2 Notes KCSE-kcse Chemistry Form 2 Notes Pdf Chemistry Form 2 Notes Pdf Download Chemistry Form 2 Past Papers Chemistry Form 2 Pdf Chemistry Form 2 Question Papers Chemistry Form 2 Questions Chemistry Form 2 Questions and Answers Chemistry Form 2 Questions and Answers Pdf Chemistry Form 2 Quiz Chemistry Form 2 Revision Notes Chemistry Form 2 Salts Chemistry Form 2 Structure and Bonding Chemistry Form 2 Summary Notes Chemistry Form 2 Syllabus Chemistry Form 2 Work Chemistry Form 3 Chemistry Form 3 and 4 Essays Chemistry Form 3 and 4 Essays Questions and Answers Chemistry Form 3 Chapter 3 Chemistry Form 3 Classification Chemistry Form 3 Diagrams Chemistry Form 3 Ecology Chemistry Form 3 Exams Chemistry Form 3 Notes Chemistry Form 3 Notes and Questions Chemistry Form 3 Notes GCSE Chemistry Form 3 Notes KCSE-kcse Chemistry Form 3 Notes Pdf Chemistry Form 3 Notes Pdf Download Chemistry Form 3 Notes Topic 1 Chemistry Form 3 Past Papers Chemistry Form 3 Pdf Chemistry Form 3 Question Papers Chemistry Form 3 Questions Chemistry Form 3 Questions and Answers Chemistry Form 3 Questions and Answers Pdf Chemistry Form 3 Questions and Answers Term 3 Chemistry Form 3 Questions and Answers+pdf Chemistry Form 3 Quiz Chemistry Form 3 Revision Notes Chemistry Form 3 Revision Questions Chemistry Form 3 Summary Notes Chemistry Form 3 Syllabus Chemistry Form 3 Syllabus Pdf Chemistry Form 3 Topics Chemistry Form 3 Work Chemistry Form 4 Chemistry Form 4 All Chapter Chemistry Form 4 Chapter 1 Conversion of Units Chemistry Form 4 Chapter 1 Exercise Chemistry Form 4 Chapter 1 Exercise and Answers Chemistry Form 4 Chapter 1 Exercise Pdf Chemistry Form 4 Chapter 1 Mind Map Chemistry Form 4 Chapter 2 Chemistry Form 4 Chapter 2 Exercise and Answers Chemistry Form 4 Chapter 2 Exercise Pdf Chemistry Form 4 Chapter 2 Experiment Chemistry Form 4 Chapter 2 Formula Chemistry Form 4 Chapter 2 Mind Map Chemistry Form 4 Chapter 2 Momentum Chemistry Form 4 Chapter 2 Notes Pdf Chemistry Form 4 Chapter 2 Objective Questions and Answers Chemistry Form 4 Chapter 2 Paper 2 Chemistry Form 4 Chapter 2 Slideshare Chemistry Form 4 Chapter 3 Chemistry Form 4 Chapter 3 Questions and Answers Chemistry Form 4 Chapter 4 Chemistry Form 4 Chapter 4 Notes Pdf Chemistry Form 4 Chapter 5 Light Questions and Answers Chemistry Form 4 Chapter 5 Notes Pdf Chemistry Form 4 Diagrams Chemistry Form 4 Exam Paper 1 Chemistry Form 4 Exams Chemistry Form 4 Exercise Chemistry Form 4 Exercise Pdf Chemistry Form 4 Module With Answer Chemistry Form 4 Note Chemistry Form 4 Notes Chemistry Form 4 Notes (Pdf) Chemistry Form 4 Notes All Chapter Pdf Chemistry Form 4 Notes and Questions Chemistry Form 4 Notes Chapter 1 Chemistry Form 4 Notes Chapter 2 Chemistry Form 4 Notes Chapter 3 Chemistry Form 4 Notes Download Chemistry Form 4 Notes Free Download Chemistry Form 4 Notes GCSE Chemistry Form 4 Notes KCSE-kcse Chemistry Form 4 Notes Pdf Chemistry Form 4 Notes Pdf Download Chemistry Form 4 Paper 2 Questions and Answers Chemistry Form 4 Past Papers Chemistry Form 4 Question Papers Chemistry Form 4 Questions Chemistry Form 4 Questions and Answers Chemistry Form 4 Questions and Answers Pdf Chemistry Form 4 Quiz Chemistry Form 4 Revision Notes Chemistry Form 4 Schemes of Work Chemistry Form 4 Summary Notes Chemistry Form 4 Syllabus Chemistry Form 4 Textbook Pdf Chemistry Form 4 Work Chemistry Form 5 Chapter 1 Exercise and Answers Chemistry Form 5 Chapter 1 Notes Pdf Chemistry Form 5 Chapter 2 Notes Pdf Chemistry Form 5 Chapter 2 Slideshare Chemistry Form 5 Chapter 3 Notes Pdf Chemistry Form 5 Notes Pdf Chemistry Form Four Book Chemistry Form Four Notes Chemistry Form Four Notes and Questions Chemistry Form Four Notes GCSE Chemistry Form Four Notes Pdf Chemistry Form Four Past Papers Chemistry Form Four Questions Chemistry Form Four Questions and Answers Chemistry Form Four Questions and Answers Pdf Chemistry Form Four Quiz Chemistry Form Four Study Notes Chemistry Form Four Syllabus Chemistry Form Four Topic 2 Chemistry Form Four Topic 4 Chemistry Form Four Topics Chemistry Form Four Work Chemistry Form One Chemistry Form One Book Chemistry Form One Book Pdf Chemistry Form One Download Topic 1 Upto 3 Chemistry Form One Exam Chemistry Form One Notes Chemistry Form One Notes and Questions Chemistry Form One Notes GCSE Chemistry Form One Notes Pdf Chemistry Form One Pdf Chemistry Form One Questions Chemistry Form One Questions and Answers Chemistry Form One Questions and Answers Pdf Chemistry Form One Questions and Their Answers Chemistry Form One Quiz Chemistry Form One Revision Question Chemistry Form One Schemes of Work Chemistry Form One Study Notes Chemistry Form One Syllabus Chemistry Form One Term Three Test Chemistry Form One to Three Notes Chemistry Form One Work Chemistry Form Three Chemistry Form Three Book Chemistry Form Three Notes Chemistry Form Three Notes and Questions Chemistry Form Three Notes GCSE Chemistry Form Three Questions and Answers Chemistry Form Three Questions and Answers Pdf Chemistry Form Three Quiz Chemistry Form Three Reproduction Chemistry Form Three Reproduction. Chemistry Form Three Study Notes Chemistry Form Three Work Chemistry Form Three-questions and Answers Chemistry Form Two Chemistry Form Two Book Chemistry Form Two Diagrams Chemistry Form Two Notes Chemistry Form Two Notes and Questions Chemistry Form Two Notes GCSE Chemistry Form Two Notes Pdf Chemistry Form Two Notes-pdf Chemistry Form Two Pdf Chemistry Form Two Questions Chemistry Form Two Questions and Answers Chemistry Form Two Questions and Answers Pdf Chemistry Form Two Quiz Chemistry Form Two Study Notes Chemistry Form Two Topics Chemistry Form Two Work Chemistry Form Two,schemes of Work Chemistry Form2 Chemistry Form2 Textbook Chemistry Game Form Four Question End Answers Chemistry Grade 10 Exam Papers Chemistry Hsc Pdf Chemistry Human Reproduction Video Chemistry IGCSE Past Papers Xtremepapers Chemistry K.c.s.e 2017 Chemistry KCSE Chemistry KCSE 2016 Chemistry KCSE 2017 Chemistry KCSE 2017 Paper 1 Chemistry KCSE Past Papers Chemistry KCSE Questions Chemistry KCSE Questions and Answer Chemistry KCSE Quizzes & Answers Chemistry KCSE Revision Chemistry KCSE Revision Notes Chemistry KCSE Setting Questions Form One and Two Chemistry Ksce 2015 Chemistry Last Year K.c.s.e Questions Chemistry Lesson Plan Form Two Chemistry Made Familiar Chemistry Mcq for Class 11 Chemistry Mcq for Class 12 Chemistry Mcq for Competitive Exams Chemistry Mcq for Competitive Exams Pdf Chemistry Mcq for Neet Pdf Chemistry Mcq for Ssc Chemistry Mcq Questions With Answers Chemistry Mcq With Answers Pdf Chemistry Mcqs for Class 12 Pdf Chemistry Mcqs With Answers Pdf Chemistry Mid Familia Form One Chemistry Mock Papers Chemistry Module Form 5 Chemistry Multiple Choice Questions and Answers Cxc Chemistry Multiple Choice Questions and Answers Pdf Chemistry Multiple Choice Questions With Answers Pdf Chemistry Note Chemistry Note Form Two All Chapters Chemistry Notes Chemistry Notes and Guestion and Answear Chemistry Notes and Syllabus Chemistry Notes Class 10 Chemistry Notes for Class 11 Pdf Chemistry Notes for Class 12 Pdf Chemistry Notes for High School Students Chemistry Notes for IGCSE 2014 Chemistry Notes Form 1 Chemistry Notes Form 1 4 Chemistry Notes Form 1 Free Download Chemistry Notes Form 1 KLB Chemistry Notes Form 1 Pdf Chemistry Notes Form 1-4 Chemistry Notes Form 1-4(1) Chemistry Chemistry Notes Form 14 Chemistry Notes Form 2 Chemistry Notes Form 2 KLB Chemistry Notes Form 2 Pdf Chemistry Notes Form 2; Chemistry Notes Chemistry Notes Form 3 Chemistry Notes Form 3 KLB Chemistry Notes Form 3 Pdf Chemistry Notes Form 4 Chemistry Notes Form 4 Chapter 2 Chemistry Notes Form 4 KLB Chemistry Notes Form 4 Pdf Chemistry Notes Form 4-pdf Chemistry Notes Form Four Chemistry Notes Form Four KLB Chemistry Notes Form Four Pdf Chemistry Notes Form One Chemistry Notes Form One KLB Chemistry Notes Form One Pdf Chemistry Notes Form One to Form Four Chemistry Notes Form Three Chemistry Notes Form Three KLB Chemistry Notes Form Three Pdf Chemistry Notes Form Two Chemistry Notes Form Two KLB Chemistry Notes Form Two Pdf Chemistry Notes Form2 Chemistry Notes IGCSE Chemistry Notes Kenya Chemistry Notes on Agroforestry Chemistry Notes Pdf Chemistry Notes: Chemistry Objective Answer Chemistry Objective Answer 2018 Chemistry Objective Questions for Competitive Exams Chemistry Objective Questions for Competitive Exams Pdf Chemistry Oral Exam Questions Chemistry Paper 1 Chemistry Paper 1 2018 Marking Rules Chemistry Paper 1 Notes Chemistry Paper 1 Questions Chemistry Paper 1 Questions and Answers Chemistry Paper 1 Topics Chemistry Paper 1 With Answers Chemistry Paper 2 Chemistry Paper 2 2017 Chemistry Paper 2 2018 Marking Rules Chemistry Paper 2 Questions and Answers Chemistry Paper 2 Questions and Answers Pdf Chemistry Paper 2 Revision Chemistry Paper 2 Topics Chemistry Paper 2018 Chemistry Paper 3 2018 Marking Rules Chemistry Paper 3 Question and Answer Chemistry Paper 3 Question Paper 2014 KCSE Chemistry Paper 3 Question Paper 2015 KCSE Chemistry Paper 3 Question Paper 2016 KCSE Chemistry Paper 3 Question Paper 2017 KCSE Chemistry Paper 3 Question Paper 2018 KCSE Chemistry Paper 3 Questions and Answers Chemistry Paper One Questions and Answers Chemistry Paper One Topics Chemistry Paper Two Qestions With Answers Chemistry Paper1 Chemistry Paper2 Chemistry Paper3 Chemistry Paper4 Chemistry Past Papers Chemistry Past Papers 2017 Chemistry Past Papers a Level Chemistry Past Papers Form 1 Chemistry Past Papers Form 2 Chemistry Past Papers Form 3 Chemistry Past Papers O Level Chemistry Pdf Download Chemistry Pp1 KCSE 2016 Chemistry Practical Book Class 12 Pdf Chemistry Practical Exam Chemistry Practicals Form One Chemistry Practicals Questions and Answers Chemistry Practice Test 9th Grade Chemistry Practice Test Answers Chemistry Practice Test Questions and Answers Chemistry Practice Test Quizlet Chemistry Predicted Questions This Year KCSE Chemistry Preparation Notes Chemistry Pretest High School Pdf Chemistry Question and Answer With Explanation Chemistry Question and Answers 2019 Chemistry Question and Answers 2020 Chemistry Question and Answers 2021 Chemistry Question and Answers 2022 Chemistry Question and Answers Note Chemistry Questions Chemistry Questions and Answers Chemistry Questions and Answers for High School Chemistry Questions and Answers for High Schools Chemistry Questions and Answers for High Schools Pdf Chemistry Questions and Answers for Secondary Schools Chemistry Questions and Answers Form 1 Chemistry Questions and Answers Form 2 Chemistry Questions and Answers Form 3 Chemistry Questions and Answers Form 4 Chemistry Questions and Answers Multiple Choice Chemistry Questions and Answers Notes Chemistry Questions and Answers O Chemistry Questions and Answers Online Chemistry Questions and Answers Pdf Chemistry Questions and Answers Pdf for Class 12 Chemistry Questions and Answers Pdf for Competitive Exams Chemistry Questions and Answers-form 2 Chemistry Questions for High School Chemistry Questions for High School Students With Answers Chemistry Questions for Senior 1 Chemistry Questions for Senior 2 Chemistry Questions for Senior 3 Chemistry Questions for Senior 4 Chemistry Questions for Senior 5 Chemistry Questions for Senior 6 Chemistry Questions for Senior Five Chemistry Questions for Senior Four Chemistry Questions for Senior One Chemistry Questions for Senior Six Chemistry Questions for Senior Three Chemistry Questions for Senior Two Chemistry Questions Form One Chemistry Questions Multiple Choice Chemistry Questions Quizlet Chemistry Questions to Ask Your Teacher Chemistry Quetion and Answer Form Four Chemistry Quetion and Answer Form One Chemistry Quetion and Answer Form Three Chemistry Quetion and Answer Form Two Chemistry Quiz for Class 9 Chemistry Quiz for Class 9 Chemistry Chemistry Quiz Questions and Answers for Class 10 Chemistry Quiz Questions and Answers for Class 10 Pdf Chemistry Quiz Questions and Answers for Class 12 Chemistry Quiz Questions and Answers for Class 9 Chemistry Quiz Questions and Answers for Class 9 Pdf Chemistry Quiz Questions and Answers for High School Chemistry Quiz Questions and Answers Multiple Choice Chemistry Quiz Questions and Answers Pdf Chemistry Quiz Questions for Class 12 Chemistry Quiz Questions for College Students Chemistry Quiz With Answers Chemistry Quiz With Answers Pdf Chemistry Quizlet Chemistry Revision Chemistry Revision a Level Chemistry Revision Chemistry Notes Chemistry Chemistry Revision Exam Chemistry Revision Examination Chemistry Revision Form One Chemistry Revision Notes Chemistry Revision Notes Chemistry Chemistry Revision Notes Form 1 Chemistry Revision Notes Form 2 Chemistry Revision Notes Form 3 Chemistry Revision Notes Form 4 Chemistry Revision Notes IGCSE Chemistry Revision Paper One Chemistry Revision Questions Chemistry Revision Questions and Answers Chemistry Revision Questions and Answers Form 1 Chemistry Revision Questions and Answers Form 2 Chemistry Revision Questions and Answers Form 3 Chemistry Revision Questions and Answers Form 4 Chemistry Revision Questions and Answers Form Four Chemistry Revision Questions and Answers Form One Chemistry Revision Questions and Answers Form Three Chemistry Revision Questions and Answers Form Two Chemistry Revision Questions Form 1 Chemistry Revision Questions Form 2 Chemistry Revision Questions Form 3 Chemistry Revision Questions Form 4 Chemistry Revision Questions Form Four Chemistry Revision Questions Form One Chemistry Revision Questions Form Three Chemistry Revision Questions Form Two Chemistry Revision Quiz Chemistry Revision Test Chemistry Secondary School Revision Chemistry Simple Notes Chemistry Spm Notes Download Chemistry Spm Notes Pdf Chemistry Spm Questions Chemistry Study Form 2 Chemistry Study Guide Chemistry Study Guide Answer Key Chemistry Study Guide Answers Chemistry Study Guide Chemistry Questions and Answers Chemistry Study Guide Ib Chemistry Study Guide Pdf Chemistry Study Guides Chemistry Study Notes Chemistry Study Notes Materials Form 1 Pdf Chemistry Study Notes Materials Form 2 3 Pdf Chemistry Study Notes Materials Form 2 Pdf Chemistry Study Notes Materials Form 3 Pdf Chemistry Study Notes Materials Form 4 Pdf Chemistry Syllabus in Kenya Chemistry Syllabus Pdf Chemistry Test 1 Quizlet Chemistry Test Questions Chemistry Test Questions and Answers Chemistry Test Questions and Answers Pdf Chemistry Topic One Form Four Chemistry Topics Form One Chemistry Unit 1 Quiz Chemistry Vol 3 Chemistry | Revision Chemistry Chemistry,form 4 Chemistry.form Four.topic Three ChemistryExam Form Three ChemistryModule Form 5 ChemistryNotes ChemistryNotes for Class 11 Pdf ChemistryNotes for Class 12 Pdf ChemistryNotes Form 1 ChemistryNotes Form 1 Free Download ChemistryNotes Form 2 ChemistryNotes Form 3 ChemistryNotes Form 3 Pdf ChemistryNotes IGCSE ChemistryNotes Pdf ChemistryPast Papers ChemistryQuestions and Answers Pdf ChemistrySimple Notes ChemistrySpm Notes Download ChemistrySpm Notes Pdf ChemistrySpm Questions ChemistryStudy Guide Answers ChemistryStudy Guide Pdf ChemistryStudy Guides Blologytextpapers Bridge Chemistry Business Past KCSE Past Papers Business Studies Form 3 Notes Pdf Business Studies Form 4 Notes Pdf C R E Form One KLB C R E Form One Oli Topic C.r.e Form 1 Notes Kenya C.r.e Form 2 Notes Kenya C.r.e Form 3 Notes C.r.e Form 3 Notes Kenya C.r.e Form 3 Pdf C.r.e Form 4 Notes Kenya C.r.e Form One Notes Pdf C.r.e Notes Form 1 C.r.e Revision Notes C.r.e Short Notes Cambridge IGCSE Chemistry Cambridge IGCSE Chemistry 3rd Edition Cambridge IGCSE Chemistry 3rd Edition Plus Cd South Asia Edition Cambridge IGCSE Chemistry Answers Cambridge IGCSE Chemistry Coursebook Pdf Download Cambridge IGCSE Chemistry Practical Workbook Cambridge IGCSE Chemistry Revision Guide Pdf Cambridge IGCSE Chemistry Study and Revision Guide 2nd Edition Pdf Cambridge IGCSE Chemistry Study and Revision Guide Pdf Cambridge IGCSE Chemistry Workbook Free Download Cambridge IGCSE Chemistry Workbook Pdf Cambridge IGCSE® Chemistry Coursebook Caucasian Chalk Circle Essay Questions Chapter 1 Introduction to Chemistry Chapter 1 Introduction to Chemistry Studies Cie a Level Chemistry Notes 2016 Cie a Level Chemistry Notes Pdf Cie Past Papers Class 10 Chemistry Chapter 1 Mcqs Class 8 Chemistry Notes KCSE-kcse College Chemistry Notes College Chemistry Practice Test College Chemistry Quiz College Chemistry Quiz Chapter 1 College Chemistry Quizlet College Chemistry Study Guide College Chemistry Study Guide Pdf College Chemistry Test Questions and Answers College Chemistry Volume 3 Pdf College ChemistryNotes Complete Chemistry for Cambridge IGCSE Complete Chemistry for Cambridge IGCSE Revision Guide Pdf County Mocks 2017 Cse Past Papers Chemistry 2017 Dl Chemistry Form 3 Pdf Kusoma Download Chemistry Form 1 Download Chemistry Form 2 Download Chemistry Form 2 Notes Download Chemistry Form 3 Download Chemistry Form 3 Notes Download Chemistry Form 4 Download Chemistry Form Four Download Chemistry Form One Download Chemistry Form Three Download Chemistry Form Two Download Chemistry Notes Form 3 Download Chemistry Notes Form One Download ChemistryNotes Form 3 Download Form Three Chemistry Notes Download Free KCSE Past Papers Chemistry Download Free KCSE Past Papers From KNEC. Download KCSE Past Papers With Answers Download KCSE Revision Notes Download KLB Chemistry Book 2 Download KLB Chemistry Book 3 Download KLB Chemistry Book 4 Download Notes of Chemistry Downloads | Chemistry | Form Four Exams | Exams Downloads | Chemistry | Form One Exams | Exams Downloads | Chemistry | Form Three Exams | Exams Downloads | Chemistry | Form Two Exams | Exams Downloads | KCSE Papers and Marking Schemes | Dvance KCSE Past Papers Easy Chemistry Questions Edexcel a Level Chemistry B Edexcel a Level Chemistry Notes Pdf Edexcel a Level Chemistry Salters Nuffield Edexcel A2 Chemistry Notes Edexcel as Chemistry Revision Guide Pdf Edexcel Chemistry A2 Revision Notes Pdf Edexcel Chemistry Unit 2 Revision Notes Edexcel GCSE Chemistry Revision Guide Pdf Edexcel IGCSE Chemistry Past Papers Edexcel IGCSE Chemistry Revision Guide Free Pdf Download Edexcel IGCSE Chemistry Revision Guide Pdf Edexcel IGCSE Chemistry Revision Guide Pdf Download Electronics Form Four Notes Energy Questions Chemistry Bowl Essay Questions and Answers KCSE Chemistry Notes Essay Questions and Answers on Betrayal in the City Essay Questions Based on Betrayal in the City Evolving World Chemistry Book 1 Pdf Evolving World Chemistry Book 4 Notes Evolving World Chemistry Book Form 1 Evolving World-history Book 3 Exam Notes for Chemistry 101 Exams KCSE Chemistry Paper 1 Questions and Answers F3 Chemistry Test Paper Find Download KCSE Past Papers With Answers Find KCSE Chemistry Essay Questions and Answers Form 1 Chemistry Exam Form 1 Chemistry Notes Form 1 Chemistry Questions and Answers Form 1 Chemistry Questions and Answers Pdf Form 1 Chemistry Revision Notes Form 1 Chemistry Summurized Revision Pdf Form 1 Chemistry Syllabus Form 1 Chemistry Test Paper Pdf Form 1 Chemistry Topics Form 1 ChemistryNotes Form 1 ChemistryQuestions and Answers Form 1 ChemistryRevision Notes Form 1 ChemistrySyllabus Form 1 ChemistryTest Paper Pdf Form 1 Past Papers Form 1 Past Papers With Answers Form 1 Revision Papers Form 1 Subjects in Kenya Form 2 Chemistry Exam Form 2 Chemistry Exam Paper Form 2 Chemistry Exam Paper 2016 Form 2 Chemistry Exam Paper Free Download Form 2 Chemistry Exam Paper With Answer Form 2 Chemistry Final Year Exam Paper 2 Form 2 Chemistry Notes Form 2 Chemistry Notes and Revision Questions Form 2 Chemistry Notes Pdf Form 2 Chemistry Past Papers Form 2 Chemistry Questions Form 2 Chemistry Questions and Answers Form 2 Chemistry Questions and Answers > Form 2 Chemistry Questions and Answers Pdf Form 2 Chemistry Revision Notes Form 2 Chemistry Short Notes Form 2 Chemistry Syllabus Form 2 ChemistryExam Paper Form 2 ChemistryExam Paper Free Download Form 2 ChemistryExam Paper With Answer Form 2 ChemistryFinal Year Exam Paper 2 Form 2 ChemistryPast Papers Form 2 ChemistryRevision Notes Form 2 ChemistryShort Notes Form 2 ChemistrySyllabus Form 2 Revision Papers Form 2 Subjects in Kenya Form 3 Chemistry Book Form 3 Chemistry Exam Form 3 Chemistry Exam Paper Form 3 Chemistry Notes Form 3 Chemistry Past Papers Form 3 Chemistry Questions Form 3 Chemistry Questions and Answers Form 3 Chemistry Questions and Answers Pdf Form 3 Chemistry Revision Notes Form 3 Chemistry Syllabus Form 3 ChemistryExam Paper Form 3 ChemistryNotes Form 3 ChemistryPast Papers Form 3 ChemistryQuestions Form 3 ChemistryQuestions and Answers Pdf Form 3 ChemistryRevision Notes Form 3 ChemistrySyllabus Form 3 C.r.e Form 3 Notes of Chemistry Topic on Fish Form 3 Past Papers Form 3 Revision Papers Form 3 Subjects in Kenya Form 4 Chemistry Exam Form 4 Chemistry Notes Form 4 Chemistry Notes Pdf Form 4 Chemistry Questions and Answers Form 4 Chemistry Questions and Answers Pdf Form 4 Chemistry Revision Notes Form 4 Chemistry Syllabus Form 4 Chemistry Topics Form 4 ChemistryNotes Form 4 ChemistryRevision Notes Form 4 ChemistrySyllabus Form 4 ChemistryTopics Form 4 Exam Papers Form 4 Revision Papers Form 4 Subjects in Kenya Form 5 Chemistry Topics Form 5 ChemistryTopics Form Five Chemistry Notes Form Five ChemistryNotes Form Four Chemistry Book Form Four Chemistry Notes Form Four Chemistry Notes Pdf Form Four Chemistry Questions and Answers Form Four Chemistry Questions and Answers Pdf Form Four Chemistry Revision Questions Form Four Chemistry Syllabus Form Four Chemistry Topics Form Four ChemistryNotes Form Four ChemistryQuestions and Answers Form Four ChemistryQuestions and Answers Pdf Form Four ChemistryTopics Form Four Notes Form Four Revision Papers Form Four Subjects in Kenya Form One Chemistry Book Form One Chemistry Examination Form One Chemistry First Topic Form One Chemistry Lesson Plan Form One Chemistry Notes Pdf Form One Chemistry Past Papers Pdf Form One Chemistry Questions Form One Chemistry Questions and Answers Form One Chemistry Questions and Answers Pdf Form One Chemistry Revision Questions Form One Chemistry Short Notes Form One Chemistry Syllabus Form One Chemistry Topics Form One ChemistryExamination Form One ChemistryPast Papers Pdf Form One ChemistryQuestions and Answers Form One ChemistryQuestions and Answers Pdf Form One ChemistryTopics Form One Exams Form One Notes of Chemistry Form One Past Papers Form One Subjects in Kenya Form One Term One Chemistry Exam Form One Term One ChemistryExam Form Three Chemistry Book Form Three Chemistry Book Pdf Form Three Chemistry Notes Form Three Chemistry Notes Pdf Form Three Chemistry Questions and Answers Form Three Chemistry Questions and Answers Pdf Form Three Chemistry Revision Questions Form Three Chemistry Syllabus Form Three Chemistry Topics Form Three ChemistryNotes Form Three ChemistryNotes Pdf Form Three ChemistryQuestions and Answers Form Three ChemistryQuestions and Answers Pdf Form Three ChemistryTopics Form Three Subjects in Kenya Form Two Chemistry Book Form Two Chemistry Cat Form Two Chemistry Examination Form Two Chemistry Notes Form Two Chemistry Notes Pdf Form Two Chemistry Past Papers Form Two Chemistry Questions and Answers Form Two Chemistry Questions and Answers Pdf Form Two Chemistry Revision Questions Form Two Chemistry Syllabus Form Two Chemistry Topics Form Two ChemistryNotes Form Two ChemistryNotes Pdf Form Two ChemistryQuestions and Answers Form Two ChemistryQuestions and Answers Pdf Form Two ChemistrySyllabus Form Two ChemistryTopics Form Two Notes Form Two Subjects in Kenya Free a-level Chemistry Revision App | Pass Your Chemistry Exams Free Chemistry Form 1 Notes Free Chemistry Notes Form 1 Free Chemistry Notes Pdf Free ChemistryNotes Pdf Free College Chemistry Practice Test Free Form1,form2,form3 Past Papers Free KCSE Past Papers Free KCSE Mocks 2015 Free KCSE Past Papers 2014 Free KCSE Past Papers KCSE Past Free KCSE Past Papers Kenya, Free KCSE Past Papers With Answers Free KCSE Questions and Answers on Chemistry Free KCSE Revision Notes Free Marking Schemes Free Mocks Online KCSE Answers Past Exams Question Papers Free Revision Papers From Three Notes Topic One KLB Fun Chemistry Questions Funny Chemistry Questions Funny Chemistry Questions and Answers Funny Chemistry Questions to Ask Funny Chemistry Quotes GCSE Chemistry Exam Questions and Answers GCSE Chemistry Past Papers GCSE Chemistry Revision GCSE Chemistry Revision Notes GCSE Chemistry Revision Notes Pdf GCSE Chemistry Revision Notes Pdf 9-1 GCSE Chemistry Revision Questions and Answers GCSE Chemistry Textbook Pdf GCSE Chemistry Topics Pass My Exams: Easy Exam Revision Notes General Chemistry Notes Pdf General Chemistry Practice Test With Answers General Chemistry Quiz General Chemistry Quiz Pdf General Chemistry Test Questions and Answers General Chemistry Test Questions and Answers Pdf General Knowledge in Chemistry Human Body Good Chemistry Questions to Ask GRE Chemistry Practice Test GRE Chemistry Subject Test Pdf Handbook of Chemistry Pdf Free Download Hard Chemistry Questions Hard Chemistry Questions and Answers Hard Chemistry Questions to Ask Your Teacher Hard Chemistry Quiz Questions Hard Form 3 Chemistry Question High School Chemistry Final Exam Doc High School Chemistry Final Exam Pdf High School Chemistry Final Exam Questions High School Chemistry Final Exam Questions and Answers High School Chemistry Notes High School Chemistry Practice Test High School Chemistry Pretest With Answers High School Chemistry Questions and Answers Pdf High School Chemistry Study Guide High School Chemistry Test Questions and Answers Pdf High School ChemistryNotes High School ChemistryStudy Guide How to Answer KCSE Chemistry Question How to Motivate a Form 4 Student How to Motivate a KCSE Candidate How to Motivate a KCSE Student How to Pass Chemistry Questions & Answers Form 1&2 | Text Book How to Revise Chemistry How to Revise Effectively for KCSE How to Study Chemistry: 5 Study Techniques to Master Chemistry Hsc Chemistry 2018 Hsc Chemistry 2019 Https://www.knec.ac.ke/ Www.knec-portal.ac.ke/ KNEC Portal: Ial Chemistry Notes Ib Chemistry Cold War Notes Ib Chemistry Notes Ib Chemistry Notes Pdf Ib Chemistry of the Americas Notes Ib Chemistry of the Americas Study Guide Ib Chemistry Paper 2 Study Guide Ib Chemistry Question Bank by Topic Ib Chemistry Study Guide Pdf Ict Notes Form 1 IGCSE Chemistry Alternative to Practical Revision IGCSE Chemistry Alternative to Practical Revision Notes IGCSE Chemistry Book IGCSE Chemistry Book Pdf Download IGCSE Chemistry Notes IGCSE Chemistry Notes 2017 Pdf IGCSE Chemistry Notes Edexcel IGCSE Chemistry Paper 2 Notes IGCSE Chemistry Paper 6 Notes IGCSE Chemistry Past Papers IGCSE Chemistry Past Papers 2014 IGCSE Chemistry Past Papers 2017 IGCSE Chemistry Pdf IGCSE Chemistry Pre Release Material 2018 IGCSE Chemistry Resources IGCSE Chemistry Revision Guide IGCSE Chemistry Revision Guide Free Download IGCSE Chemistry Revision Guide Pdf Download IGCSE Chemistry Revision Notes Pdf IGCSE Chemistry Revision Worksheets IGCSE Chemistry Workbook Pdf IGCSE Chemistry Znotes IGCSE ChemistryPast Papers IGCSE Notes Chemistry Importance of Agroforestry Inorganic Chemistry Multiple Choice Questions With Answers Pdf Inorganic Chemistry Questions and Answers Pdf Interesting Chemistry Questions Interesting Chemistry Questions and Answers Interesting Questions to Ask About Chemistry Intro to Chemistry Quiz Introduction of Chemistry Form One Introduction to Chemistry Introduction to Chemistry Notes Introduction to Chemistry Pdf Introduction to ChemistryNotes Is Agroforestry Sustainable? K.c.s.e Answers Chemistry Paper One 2018 K.c.s.e Chemistry 2017 K.c.s.e Chemistry 2018 K.c.s.e Chemistry Paper 1 2017 K.c.s.e Mocks 2018 K.c.s.e Papers 2015 K.c.s.e Papers 2016 K.c.s.e Past Papers 2014 K.c.s.e.Chemistry Paper 2 Year 2018 K.c.s.e.results 2018 for Busia County K.l.b Chemistry Form 3 K.l.b Chemistry Notes K.l.b ChemistryNotes Kasneb Past Papers for Colleges Chemistry Past Papers KCSE 2010 Marking Scheme KCSE 2010 Past Papers KCSE 2011 Chemistry Paper 1 KCSE 2011 Marking Scheme KCSE 2012 Chemistry Paper 2 Marking Scheme KCSE 2012 Marking Schemes KCSE 2013 Chemistry Paper 1 KCSE 2013 Marking Scheme KCSE 2013 Marking Scheme Pdf KCSE 2014 KCSE 2015 Chemistry Paper 2 KCSE 2015 Chemistry Paper 3 KCSE 2015 Marking Scheme KCSE 2015 Past Papers KCSE 2016 Chemistry Paper 1 KCSE 2016 Chemistry Paper 2 KCSE 2017 Chemistry Paper 1 KCSE 2017 Chemistry Paper 2 KCSE 2017 Hostory Papers With Answers.com KCSE 2017 Marking Scheme KCSE 2017 Papers KCSE 2017 Papers and Marking Scheme KCSE 2017 Papers Pdf KCSE 2017 Past Papers KCSE 2017 Prediction Pdf KCSE 2018 Chemistry and Answers KCSE 2018 Chemistry Prediction KCSE 2018 Leakage KCSE 2018 Marking Scheme KCSE 2018 Papers KCSE 2018 Prediction Pdf KCSE 2018 Predictions KCSE 2018 Questions KCSE 2018 Questions and Answers KCSE 2019 Leakage Chemistry KCSE 2019 Marking Scheme KCSE 2019 Questions KCSE 2019 Questions and Answers KCSE 2020 Questions KCSE 2020 Questions and Answers KCSE Answers KCSE Answers Past Exams Question Papers Downloads | KCSE Chemistry 2011 KCSE Chemistry 2016 KCSE Chemistry Diagramsbiology Revision Tips KCSE Chemistry Essay Questions and Answers KCSE Chemistry Essay Questions and Answers Pdf KCSE Chemistry Essays KCSE Chemistry Essays Pdf KCSE Chemistry Marking Schemes KCSE Chemistry Notes KCSE Chemistry Notes Pdf KCSE Chemistry Notes, Syllabus, Questions, Answers KCSE Chemistry Paper 1 KCSE Chemistry Paper 1 2011 KCSE Chemistry Paper 1 2012 KCSE Chemistry Paper 1 2013 KCSE Chemistry Paper 1 2015 KCSE Chemistry Paper 1 2016 KCSE Chemistry Paper 1 2017 KCSE Chemistry Paper 1 2017 Pdf KCSE Chemistry Paper 1 Questions and Answers KCSE Chemistry Paper 2 KCSE Chemistry Paper 2 2012 KCSE Chemistry Paper 2 2012 KCSE Chemistry Paper 2 2015 KCSE Chemistry Paper 2 2013 KCSE Chemistry Paper 2 2014 KCSE Chemistry Paper 2 2015 KCSE Chemistry Paper 2 2016 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 3 KCSE Chemistry Paper 3 2012 KCSE Chemistry Paper 3 2016 KCSE Chemistry Paper 3 2017 KCSE Chemistry Paper 3 Past Papers KCSE Chemistry Past Papers KCSE Chemistry Past Papers and Answers KCSE Chemistry Past Papers Pdf KCSE Chemistry Practical KCSE Chemistry Practical 2015 KCSE Chemistry Practical 2016 KCSE Chemistry Practical Past Papers KCSE Chemistry Practicals KCSE Chemistry Practicals KCSE Chemistry Paper 1 KCSE Chemistry Question and Answer KCSE Chemistry Questions and Answers KCSE Chemistry Questions and Answers Ap Chemistry KCSE Chemistry Revision KCSE Chemistry Revision Notes KCSE Chemistry Revision Papers KCSE Chemistry Revision Questions KCSE Chemistry Revision Questions and Answers KCSE Chemistry Syllabus KCSE ChemistryNotes KCSE ChemistryPaper 1 KCSE ChemistryPaper 2 KCSE ChemistryPaper 2 Pdf KCSE ChemistrySyllabus KCSE Business Paper 1 2016 KCSE Business Past Papers KCSE Business Studies Past Papers KCSE Essay Questions in Betrayal in the City KCSE Essays KCSE Exam Papers 2018 KCSE Exam Papers Answers KCSE Form 1 Chemistry Revision KCSE Form 2 Chemistry Revision KCSE Form 3 Chemistry Revision KCSE Form 4 Chemistry Revision KCSE Form Four Chemistry Revision KCSE Form One Chemistry Revision KCSE Form Three Chemistry Revision KCSE Form Two Chemistry Revision KCSE KCSE Past Papers KNEC KCSE Leakage KCSE Leakage Chemistry KCSE Made Familiar Chemistry KCSE Made Familiar Chemistry Pdf KCSE Marking Scheme 2016 KCSE Marking Schemes KCSE Marking Schemes 2017 KCSE Marking Schemes Pdf KCSE Mock Exams KCSE Mock Papers 2015 KCSE Mock Papers 2017 KCSE Mock Papers 2018 KCSE Mock Papers Pdf KCSE Mock Papers Pdf 2018 KCSE Mock Papers Pdf KCSE Past Papers KCSE Mocks 2017 KCSE Mocks 2018 KCSE Notes KCSE Online Notes KCSE Online Past Papers KCSE Online Registration KCSE Papers 2015 KCSE Papers and Marking Schemes | Exams KCSE Past Papers KCSE Past Papers 2007 KCSE Past Papers 2009 KCSE Past Papers 2010 KCSE Past Papers 2011 KCSE Past Papers 2011 Pdf KCSE Past Papers 2012 KCSE Past Papers 2013 KCSE Past Papers 2013knec KCSE Past Papers 2014 KCSE Past Papers 2014 Pdf KCSE Past Papers 2015 KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2015 Pdf KCSE Past Papers 2016 KCSE Past Papers 2016 Pdf KCSE Past Papers 2017 KCSE Past Papers 2017 Pdf KCSE Past Papers 2018 KCSE Past Papers Chemistry KCSE Past Papers Chemistry and Answers KCSE Past Papers Chemistry Pdf KCSE Past Papers Chemistry With Answers KCSE Past Papers Chemistryand Answers KCSE Past Papers Business Studies and Answers KCSE Past Papers KCSE and Answers KCSE Past Papers KCSE and Answers Free Mocks Online KCSE Past Papers Marking Scheme KCSE Past Papers Pdf Download KCSE Past Papers Pdf Download KCSE 2013 KCSE Past Papers With Answers KCSE Past Papers Woodwork and Answers KCSE Prediction 2017 KCSE Prediction 2018 KCSE Prediction 2018 Pdf KCSE Prediction Papers 2018 KCSE Prediction Questions KCSE Prediction Questions 2018 KCSE Prediction Questions and Answers KCSE Questions KCSE Questions and Answers KCSE Questions and Answers. KCSE Questions on Chemistry KCSE Results, Online Registration, KCSE Result Slip. KCSE Revision KCSE Revision Notes KCSE Revision Notes Chemistry KCSE Revision Notes Pdf KCSE Revision Papers KCSE Revision Papers 2014 KCSE Revision Papers With Answers KCSE Revision Question for Chemistry KCSE Revision Questions KCSE Revision Questions and Answers KCSE Revision | Secondary School | Text Books | Text Book Centre KCSE Syllabus Pdf KCSE Trial 2017 KCSE Trial Exams 2017 Kenya Secondary School Chemistry Syllabus Kenya Secondary School Chemistry Syllabus Pdf Kenya Secondary School ChemistrySyllabus Pdf Kenya Secondary School Syllabus Pdf Kenya-kcse-christian Religious Education Syllabus Kenyaplex KCSE Past Papers Kenyaplex Past Papers for Secondary KLB Chemistry Book 1 Download KLB Chemistry Book 1 Notes KLB Chemistry Book 1 Pdf KLB Chemistry Book 2 KLB Chemistry Book 2 Notes KLB Chemistry Book 2 Notes Pdf KLB Chemistry Book 2 Pdf KLB Chemistry Book 3 Notes KLB Chemistry Book 3 Pdf KLB Chemistry Book 3 Pdf Download KLB Chemistry Book 4 Notes KLB Chemistry Book 4 Pdf KLB Chemistry Book 4 Pdf Download KLB Chemistry Book 4 Topics KLB Chemistry Book One KLB Chemistry Form 1 KLB Chemistry Form 1 Notes KLB Chemistry Form 1 Pdf KLB Chemistry Form 2 KLB Chemistry Form 2 Book KLB Chemistry Form 2 Notes KLB Chemistry Form 2 Pdf KLB Chemistry Form 2 Pdf Download KLB Chemistry Form 2 Schemes of Work KLB Chemistry Form 3 KLB Chemistry Form 3 Notes KLB Chemistry Form 3 Notes Pdf KLB Chemistry Form 3 Pdf KLB Chemistry Form 3 Pdf Download KLB Chemistry Form 4 KLB Chemistry Form 4 Notes KLB Chemistry Form 4 Pdf KLB Chemistry Form Four KLB Chemistry Form Four Notes KLB Chemistry Form One KLB Chemistry Form One Notes KLB Chemistry Form Three KLB Chemistry Form Three Notes KLB Chemistry Form Two KLB Chemistry Form Two Notes KLB Chemistry Notes KLB Chemistry Notes Form 4 KLB Chemistry Pdf KLB ChemistryNotes KLB ChemistryNotes Form 4 KLB ChemistryPdf KNEC Chemistry Syllabus KNEC Examiners Portal KNEC Website KNEC Ict Past Papers KNEC Past Papers for Colleges KNEC Past Papers Free Download KNEC Past Papers Free Downloads KNEC Past Papers Pdf KNEC Portal Confirmation KNEC Portal KCSE Results KNEC Portal KNEC Past Papers for Colleges Kasneb Past Papers KNEC Revision Papers KNEC Technical Exams Past Papers Kusoma Chemistry Notes Kusoma Chemistry Notes Pdf Kusoma Notes Chemistry Kusoma.co.ke Kusoma.com Past Papers Learner Guide for Cambridge IGCSE Chemistry Longhorn Chemistry Book 3 Pdf Made Familiar Chemistry Made Familiar Chemistry Pdf Made Familiar Chemistry Questions Maktaba Tetea Notes Marking Scheme KCSE Chemistry Past Papers Math Form2 Note Mcqs About Gaseous Exchange Middle School Chemistry Bowl Chemistry Questions Mock Past Papers 2017 Mock Past Papers With Answers Mokasa Mock 2017 More Than 1800 Chemistry Questions and Answers to Help You Study Multiple Choice Questions on Chemistry Necta Chemistry Past Papers Necta Chemistry Practicals Necta ChemistryPast Papers Necta ChemistryPracticals Necta Form Four Past Papers Necta Past Papers Form 4 Necta Past Papers Form 4 2016 Necta Past Papers Form Six Necta Past Papers Form Two Necta Questions and Answers Necta Review Questions Notes Chemistry Form 1 Notes Chemistry Form 2 Notes Chemistry Form 3 Notes Chemistry Form 3 Notes Pdf Notes Chemistry Form 3 Syllabus Notes Chemistry Form 4 Syllabus Notes on Chemistry Studies Notes Za Chemistry 4m 2 Notes Za Chemistry Form One Notes Za Chemistry Form Three O Level Chemistry Practical Experiments O Level Chemistry Questions and Answers Pdf Orm Three Chemistry Notes Page Navigation Papacambridge Chemistry IGCSE Papers KNEC KCSE Online Past Papers KNEC KCSE Results Past Papers Past KCSE Papers Past Paper Questions by Topic Chemistry Past Papers 2014 Past Papers in Kenya Pdf Chemistry Form 3 Pdf Chemistry Notes Pdf Chemistry Notes Form 1 Pdf Chemistry Notes Form 2 Pdf Chemistry Notes Form 3 Pdf Chemistry Notes Form 4 Pdf Chemistry Notes Form Four Pdf Chemistry Notes Form One Pdf Chemistry Notes Form Three Pdf Chemistry Notes Form Two Pdf Form 1 Chemistry Questions and Answers Pdf Form 2 Chemistry Questions and Answers Pdf Form 3 Chemistry Questions and Answers Pdf Form 4 Chemistry Questions and Answers Pdf Form Four Chemistry Questions and Answers Pdf Form One Chemistry Questions and Answers Pdf Form Three Chemistry Questions and Answers Pdf Form Two Chemistry Questions and Answers Pdf Free KCSE Past Papers and Marking Schemes Pdf” Revision Questions Chemistry Form 1 Practical Chemistry Experiments Pdf Practical Chemistry Question and Answer Pdf Pre Mocks 2018 Preliminary Chemistry Primary and Secondary Tillage Implements Ppt Pte KNEC Past Papers Questions and Answers Pdf Chemistry Form 1 Questions and Answers Pdf Chemistry Form 2 Questions and Answers Pdf Chemistry Form 3 Questions and Answers Pdf Chemistry Form 4 Questions and Answers Pdf Chemistry Form Four Questions and Answers Pdf Chemistry Form One Questions and Answers Pdf Chemistry Form Three Questions and Answers Pdf Chemistry Form Two Questions Based to Introduction to Chemistry Questions on Gaseous Exchange in Humans Questions on Introduction to Chemistry Questions to Ask in Chemistry Class Questions to Confuse Your Chemistry Teacher Quizlet Chemistry Test Quizlet Test Questions Qustions in Chemistry and Answers Revision Revision Chemistry Notes and Questions? Revision Quiz for Chemistry for Form Three S.1 Chemistry Questions S.2 Chemistry Questions S.3 Chemistry Questions S.4 Chemistry Questions Sample Essays on Betrayal in the City School Chemistry Notes Secondary Chemistry Notes Secondary Chemistry Notes Pdf Secondary ChemistryNotes Pdf Senior 1 Chemistry Notes Senior 2 Chemistry Notes Senior 3 Chemistry Notes Senior 4 Chemistry Notes Senior 5 Chemistry Notes Senior 6 Chemistry Notes Senior Five Chemistry Notes Senior Four Chemistry Notes Senior One Chemistry Notes Senior Six Chemistry Notes Senior Three Chemistry Notes Senior Two Chemistry Notes Simple Scientific Questions Smart Questions to Ask a Chemistry Teacher Snab Chemistry Revision Notes Southwest Mock Paper 2 2016 Chemistry Only Spm Chemistry Revision Notes Spm Notes Success Chemistry Spm Pdf Success ChemistrySpm Pdf Summary of Chemistry Form 3 Tahossa Past Papers To Motivate a Form 4 KCSE Student To Motivate a Form 4 Student Topical Revision Material Tricky Chemistry Questions and Answers Tricky Chemistry Questions for Adults Tricky Chemistry Questions With Answers Tricky Chemistry Quiz Questions Two Chemistry Revision Questions University Chemistry Volume 3 Openstax University Chemistry Volume 3 Pdf University Chemistry Volume 4 Pdf Ur Revision Guide IGCSE Chemistry What Are the Types of Gametes Working of Excretory System Www.Chemistry Form One Notes.com Www.Chemistry From One KLB.com Www.form 1 Chemistry.com Www.form 2 Chemistry.com Www.form 3 Chemistry.com Www.form 4 Chemistry.com Www.form Four Chemistry.com Www.form One Chemistry.com Www.form Three Chemistry.com Www.form Two Chemistry.com Www.kusoma Notes Www.kusoma Revision Materials Www.kusoma.co.ke Chemistry Notes Xtremepapers IGCSE Chemistry Year 11 Chemistry Z Notes Chemistry IGCSE Znotes as Chemistry Acids and Bases Chem Revision Questions Chemistry Notes Combine Form 1, 2, 3 and 4 Chemistry Notes Form 3 A Level Chemistry Questions and Answers Pdf All Chemistry Questions and Answers Pdf,ppt Chemistry Exam Questions and Answers Pdf Chemistry Multiple Choice Questions and Answers Pdf Chemistry Questions and Answers for High Schools Pdf Chemistry Notes Form 1-4 Pdf Free High School Notes Kenya Free Kcse Revision Notes General Chemistry Test Questions and Answers Pdf High School Chemistry Multiple Choice Questions and Answers Pdf Kcse Chemistry Revision Notes Pdf Kcse Revision Books Pdf Kcse Revision Notes Pdf Kenya Secondary School Notes Pdf Notes of Form 123 and 4 All Subject Chemistry Notes Form 1 Free Download Advice to KCSE Candidates Best Revision Books for KCSE How to Pass an Exam Successfully How to Pass KCSE 2018 How to Pass KCSE 2019 How to Pass KCSE Chemistry Paper How to Pass KCSE Chemistry How to Pass KCSE Chemistry How to Pass KCSE 2019 K.c.s.e Chemistry Paper 1 2017 KCSE 2019 Prediction KCSE Prediction 2019 KCSE Revision Tips KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 2 2018 KCSE Chemistry Past Papers Pdf KCSE Past Papers 2012 KCSE Past Papers 2017 Pdf KCSE Past Papers 2018 KCSE Past Papers of Chemistry Pp2 KCSE Chemistry Past Papers Pdf Chemistry KCSE Papers With Their Marking Schemes Chemistry Paper 1 and Answers KCSE 2017 Papers and Marking Scheme KCSE 2019 Papers and Marking Scheme KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Past Papers and Answers KCSE Marking Schemes Pdf KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2019 Marking Schemes Chemistry KCSE Papers With Their Marking Schemes Chemistry Paper 1 and Answers KCSE 2017 Papers and Marking Scheme KCSE 2019 Papers and Marking Scheme KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 1 2019 Past Papers KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Paper 2 2019 Past Papers KCSE Chemistry Paper 3 2019 Past Papers KCSE Chemistry Past Papers and Answers KCSE Marking Schemes Pdf KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2019 Marking Schemes KCSE Past Papers Chemistry Paper 1 2019 KCSE Past Papers Chemistry Paper 2 2019 KCSE Past Papers Chemistry Paper 3 2019 Past Papers KCSE Chemistry Paper 1 2019 Past Papers KCSE Chemistry Paper 2 2019 Past Papers KCSE Chemistry Paper 3 2019 Chemistry Form 1 Questions and Answers Chemistry Paper 1 2018 Chemistry Paper 2 2018 Chemistry Paper 1 2019 Chemistry Paper 2 2019 Chemistry Paper 1 Notes Chemistry Paper 1 Questions and Answers Pdf Chemistry Paper 2 Questions and Answers Chemistry Questions and Answers Form 2 KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 1 2017 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2019 Most Tested KCSE Chemistry Questions 4m1 Notes Viusasa 4m2 Notes Viusasa 4m3 Notes Viusasa 4m4 Notes Viusasa Download on Viusasa – Download Now for Free Elimu – Viusasa Elimu Library | Notes, Exams, Lesson Plans, Schemes Elimu Online High School Notes – Revision Materials for Kenyan Schools Https //www.viusasa.com/elimu Kenya Notes Viusasa Elimu Form 1 Notes Viusasa Elimu Form 2 Notes Viusasa Elimu Form 3 Notes Viusasa Elimu Form 4 Notes Viusasa Elimu Form Four Notes Viusasa Elimu Form One Notes Viusasa Elimu Form Three Notes Viusasa Elimu Form Two Viusasa Viusasa Education Viusasa Elimu Viusasa Elimu Class 6 Viusasa Elimu Form 1 Viusasa Elimu Form 1 Notes Viusasa Elimu Form 2 Viusasa Elimu Form 2 Notes Viusasa Elimu Form 3 Viusasa Elimu Form 3 Notes Viusasa Elimu Form 4 Viusasa Elimu Form 4 Notes Viusasa Elimu Form Four Viusasa Elimu Form Four Notes Viusasa Elimu Form One Viusasa Elimu Form One Notes Viusasa Elimu Form Three Viusasa Elimu Form Three Notes Viusasa Elimu Form Two Viusasa Elimu Form Two Notes Viusasa High School Notes – Revision Materials for Kenyan Schools Viusasa Notes Chemistry Mock Papers Chemistry Paper 1 2019 Chemistry Paper 1 Notes Chemistry Paper 1 Questions and Answers Chemistry Paper 1 Questions and Answers Pdf Chemistry Paper 2 Form 3 Chemistry Paper 2 Notes Chemistry Paper 2 Questions and Answers Pdf Chemistry Past Papers Chemistry Past Papers Pdf Chemistry Questions and Answers Pdf Chemistry Revision Questions and Answers Pdf Chemistry Revision Questions Pdf Common Exam Questions in Chemistry Paper 1 Common Exam Questions in Chemistry Paper 2 Common Test Questions in Chemistry Paper 1 Common Tested Questions in Chemistry Paper 1 Commonly Tested Questions in Chemistry Paper 1 K.c.s.e.Chemistry Questions and Answers KCSE 2019 Chemistry Paper 1 Marking Scheme KCSE 2019 Chemistry Paper 2 KCSE 2019 Chemistry Paper 2 Marking Scheme KCSE 2020 Prediction Questions and Answers KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2016 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Questions and Answers Most Tested Questions in Chemistry Paper 1 Most Tested Questions in Chemistry Paper 2 Mostly Tested Questions in Chemistry Paper 1 Mostly Tested Questions in Chemistry Paper 2 Chemistry Form 4 Chemistry Revision Questions Form 1 Combined Science Notes Form 1 Form 4 Notes High Flyer Series Chemistry Form 1-4 Kcse Past Papers Chemistry With Answers Chemistry Notes Download Secondary Chemistry Notes Pdf Water and Hydrogen Form 1 Notes High Flyer Series KCSE Revision in Chemistry High Flyer Series KCSE Revision Chemistry Form 1-4 Revised A Level Chemistry Notes Uganda Pdf Download Buddo Junior School Holiday Work Chemistry Notes for a Level Pdf Chemistry Notes Form 1 Chemistry Notes Form 3 the Mole Chemistry Notes Form 4 Chemistry Notes O Level Chemistry Notes O Level Uganda Chemistry Notes O Level Uganda Pdf Chemistry Notes Pdf Download Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Questions and Answers Form Three Chemistry Syllabus Pdf Gayaza High School Chemistry Notes Gayaza High School Chemistry Notes Pdf Gayaza High School Chemistry Past Papers Gayaza High School Chemistry Past Papers Answers Gayaza High School Chemistry Past Papers Questions and Answers Gayaza High School E Learning Gayaza High School Elearning Platform Gayaza High School Examinations Gayaza High School Holiday Work Gayaza High School Notes Gayaza High School Notes 2021 Gayaza High School Notes 2022 Gayaza High School Notes 2023 Gayaza High School Notes Pdf Gayaza Junior School E Learning Platform Gayaza Junior School Holiday Work Gayaza O Level Chemistry Notes Home › Gayaza High School | Elearning Platform Kabojja Junior School Holiday Work Pdf Klb Chemistry Book 3 Klb Chemistry Form 3 Teachers Guide Klb Chemistry Notes List of Ugandan E-learning Platforms for Students Mirembe Junior School Holiday Work Ntare School Past Papers O Level Chemistry Notes Free Download O Level Chemistry Notes in Uganda O Level Chemistry Notes Pdf O Level Chemistry Notes Uganda Pdf O Level Chemistry Notes Uganda Pdf Download O Level General – Home › Gayaza High School | Elearning S.1 Chemistry Notes | Standard High School Zzana S.3 Chemistry Notes S.4 Chemistry 2 Notes | Standard High School Zzana Seeta High School Elearning Seeta High School Holiday Work Seeta High School Notes Seeta High School Notes Pdf Seeta High School Past Papers Seeta High School Past Papers Pdf Senior 1 Chemistry Notes Senior 1 Chemistry Notes in Uganda Senior 1 Chemistry Notes Uganda Senior 1 Chemistry Questions Senior 1 Exams Senior 1 Work Senior 1 Work 2020 Senior 1 Work 2020 Uganda Senior 2 Chemistry Notes Senior 2 Chemistry Notes in Uganda Senior 2 Chemistry Notes Uganda Senior 2 Chemistry Questions Senior 2 Exams Senior 2 Work Senior 2 Work 2020 Senior 2 Work 2020 Uganda Senior 3 Chemistry Notes Senior 3 Chemistry Notes in Uganda Senior 3 Chemistry Notes Uganda Senior 3 Chemistry Questions Senior 3 Exams Senior 3 Work Senior 3 Work 2020 Senior 3 Work 2020 Uganda Senior 4 Chemistry Notes Senior 4 Chemistry Notes in Uganda Senior 4 Chemistry Notes Uganda Senior 4 Chemistry Questions Senior 4 Exams Senior 4 Work Senior 4 Work 2020 Senior 4 Work 2020 Uganda Senior Four Chemistry Notes Senior Four Chemistry Notes in Uganda Senior Four Chemistry Notes Uganda Senior Four Chemistry Questions Senior Four Exams Senior Four Work Senior Four Work 2020 Senior Four Work 2020 Uganda Senior One Chemistry Notes Senior One Chemistry Notes in Uganda Senior One Chemistry Notes Uganda Senior One Chemistry Questions Senior One Exams Senior One Work Senior One Work 2020 Senior One Work 2020 Uganda Senior Three Chemistry Notes Senior Three Chemistry Notes in Uganda Senior Three Chemistry Notes Uganda Senior Three Chemistry Questions Senior Three Exams Senior Three Work Senior Three Work 2020 Senior Three Work 2020 Uganda Senior Two Chemistry Notes Senior Two Chemistry Notes in Uganda Senior Two Chemistry Notes Pdf Senior Two Chemistry Notes Uganda Senior Two Chemistry Questions Senior Two Exams Senior Two Work Senior Two Work 2020 Senior Two Work 2020 Uganda St Mary’s Kitende E Learning Standard High School Notes Standard High School Zana a Level Notes Standard High School Zana Com Notes Standard High School Zana E Learning Standard High School Zana E Learning Platform Standard High School Zana E-learning Platform Standard High School Zana Notes for Senior Two Standard High School Zana Notes Pdf Standard High School Zana Website Standard High Zana Notes Uace Chemistry Notes Uace Chemistry Notes Pdf Uce Chemistry Notes Pdf Uce Chemistry Notes Pdf Download Uce Past Papers Uganda Secondary Schools E-learning Platform Uneb Marking Guides Pdf Uneb Past Papers and Answers Pdf Air and Combustion Form 1 Notes Chemistry Form 1 Notes Pdf Free Download Chemistry Form One Questions and Answers Chemistry Notes Form 1 Easy Elimu Chemistry Notes Form 1-4 Pdf Chemistry Notes Form 1-4 Pdf Download Free High School Chemistry Notes Pdf Klb Chemistry Form 1 Notes Pdf Download Notes of Form One Chemistry Laboratory and Drawings Chemistry Form 1 Questions and Answers Pdf Download Chemistry Notes Form 1-4 Chemistry Form 1 Questions and Answers Pdf Form 1 Chemistry Topical Questions Form 1 Chemistry Exam Paper With Answer Pdf Form 1 Exams 2020 Form 1 Chemistry Past Papers Form 1 Chemistry Revision Papers With Answers Sample Chemistry Test for Form One Exams Ntare School Past Papers Seeta High School Notes Pdf Seeta High School Past Papers Seeta High School Past Papers Pdf St Mary’s Kitende Past Papers Uce Chemistry Notes Pdf Uce Past Papers Uneb Marking Guides Pdf Uneb Past Papers and Answers Pdf Chemistry Book 3 Download Chemistry Form 3 Questions and Answers Chemistry Form 3 Syllabus Chemistry Form 3 Topics Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Notes Form Three Chemistry Notes Pdf Download Form 3 Chemistry Exam Form 3 Chemistry Notes on Pdf Revision Chemistry Revision a Level Chemistry Revision Notes – Chemistry S.1-Chemistry-notes S.1-Chemistry-notes Uganda S.2-Chemistry-notes S.2-Chemistry-notes Uganda S.3-Chemistry-notes S.3-Chemistry-notes Uganda S.4-Chemistry-notes S.4-Chemistry-notes Uganda S.5-Chemistry-notes S.5-Chemistry-notes Uganda S.6-Chemistry-notes S.6-Chemistry-notes Uganda S1 Chemistry Notes Term 1 S1 Chemistry Notes Term 2 S1 Chemistry Notes Term 3 S2 Chemistry Notes Term 1 S2 Chemistry Notes Term 2 S2 Chemistry Notes Term 3 S3 Chemistry Notes Term 1 S3 Chemistry Notes Term 2 S3 Chemistry Notes Term 3 S4 Chemistry Notes Term 1 S4 Chemistry Notes Term 2 S4 Chemistry Notes Term 3 Senior 3 Chemistry Notes Senior 3 Chemistry Notes Uganda Senior Three Chemistry Notes Senior Three Chemistry Notes Uganda Chemistry Form 1 the Cell Chemistry Form One Notes Free – Education News Chemistry Notes Form 1-4 Chemistry Questions and Answers Form 1 – Chemistry Form One Chemistry Questions and Answers Form 2 – Chemistry Form Two Chemistry Questions and Answers Form 3 – Chemistry Form Three Chemistry Questions and Answers Form 4 – Chemistry Form Four Chemistry Questions and Answers Senior 1 – Chemistry Senior One Chemistry Questions and Answers Senior 2 – Chemistry Senior Two Chemistry Questions and Answers Senior 3 – Chemistry Senior Three Chemistry Questions and Answers Senior 4 – Chemistry Senior Four Chemistry Topic by Topic Questions and Answers Combined Science Notes Form 1 Form 1 Chemistry Revision Questions and Answers Form 1 Chemistry Topical Questions Form 1 Revision Papers With Answers Form 2 Chemistry Revision Questions and Answers Form 3 Chemistry Revision Questions and Answers Form Four Chemistry Revision Questions and Answers Form One Chemistry Revision Questions and Answers Form Three Chemistry Revision Questions and Answers Form Two Chemistry Revision Questions and Answers Free Chemistry Form 1 Notes Free Chemistry Notes Introduction to Chemistry Form One Kcse Past Papers: Chemistry Form 1 Kcse Past Papers: Chemistry Form 1 Topical Questions Kcse Past Papers: Chemistry Form 1 Topical Questions and Answers Most Tested Areas in Chemistry Kcse Most Tested Areas in Chemistry Kcse Exams Most Tested Areas in Chemistry Uneb Most Tested Areas in Form 1 in Chemistry Most Tested Areas in Form 2 in Chemistry Most Tested Areas in Form 3 in Chemistry Most Tested Areas in Form 4 in Chemistry Most Tested Areas in Form Four in Chemistry Most Tested Areas in Form One in Chemistry Most Tested Areas in Form Three in Chemistry Most Tested Areas in Form Two in Chemistry Most Tested Areas in Kcse Chemistry Most Tested Areas in Kcse Chemistry Exams Most Tested Areas in Senior 1 in Chemistry Most Tested Areas in Senior 2 in Chemistry Most Tested Areas in Senior 3 in Chemistry Most Tested Areas in Senior 4 in Chemistry Most Tested Areas in Senior Four in Chemistry Most Tested Areas in Senior One in Chemistry Most Tested Areas in Senior Three in Chemistry Most Tested Areas in Senior Two in Chemistry Most Tested Areas in Uneb Chemistry Revision Notes Chemistry Form 1 – Free Kcse Past Papers Who Was Chemistry Champion KCSE 2019 Top 100 Students in Chemistry KCSE 2019 Best 100 Students in Chemistry KCSE 2019 Top Student in Chemistry KCSE Best Student in Chemistry KCSE Who Was Chemistry Champion KCSE 2020 Top 100 Students in Chemistry KCSE 2020 Best 100 Students in Chemistry KCSE 2020 Names of Best Chemistry Students KCSE Names of Top Chemistry Students KCSE High School Chemistry Notes Pdf Best High School Chemistry Notes Pdf Comprehensive High School Chemistry Notes Pdf Klb Chemistry Form 1 Book Pdf Chemistry Form 1 Best Notes Chemistry Form 1 Notes Pdf Chemistry Form 1 Pressure Chemistry Form 1 Questions and Answers Chemistry Form 1 Questions and Answers Pdf Download Chemistry Form 2 Best Notes Chemistry Form 3 Best Notes Chemistry Form 4 Best Notes Chemistry Form Four Best Notes Chemistry Form One Best Notes Chemistry Form Three Best Notes Chemistry Form Two Best Notes Chemistry Notes Form 1 to 4 Chemistry Notes Pdf